4: The Aldol Condensation – Preparation of Chalcones (Experiment)
- Page ID
- 126797
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
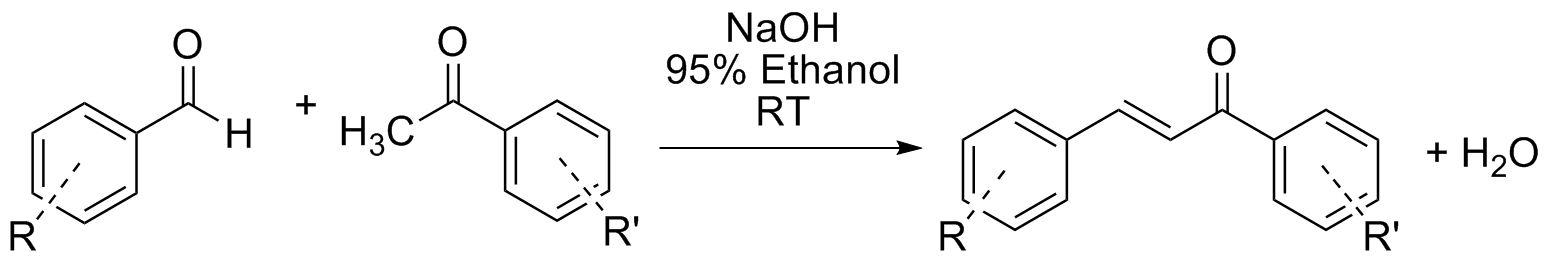
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)All of the possible products in experiment 4 belong to a particular class of molecules called chalcones. Chalcones (kal-cones) are biosynthesized in plants and have impressive biological activity including ant-oxidant, anti-fungal, anti-bacterial, anti-tumor, and anti-inflammatory properties. All chalcones share the same basic structural skeleton, which is composed of an \(\alpha, \beta\)-unsaturated ketone linking two aromatic rings. Many can be isolated from plant material and can also be synthetically prepared using the aldol condensation reaction. Neves, M.P. et al Bioorg. Med. Chem. 2012, 20, 25
Reaction Scheme
Techniques used
- melting point
- recrystallization
- gravity & vacuum filtration
- thin layer chromatography
- and infrared spectroscopy
Objective
Experiment 4 is a group experiment. You will work in a small group to design and carry out experiments in an attempt to answer one of the focus questions below. Your objective is to form and test a hypothesis in response to the focus question. The general procedure is provided below and should be adapted appropriately.
Focus Questions
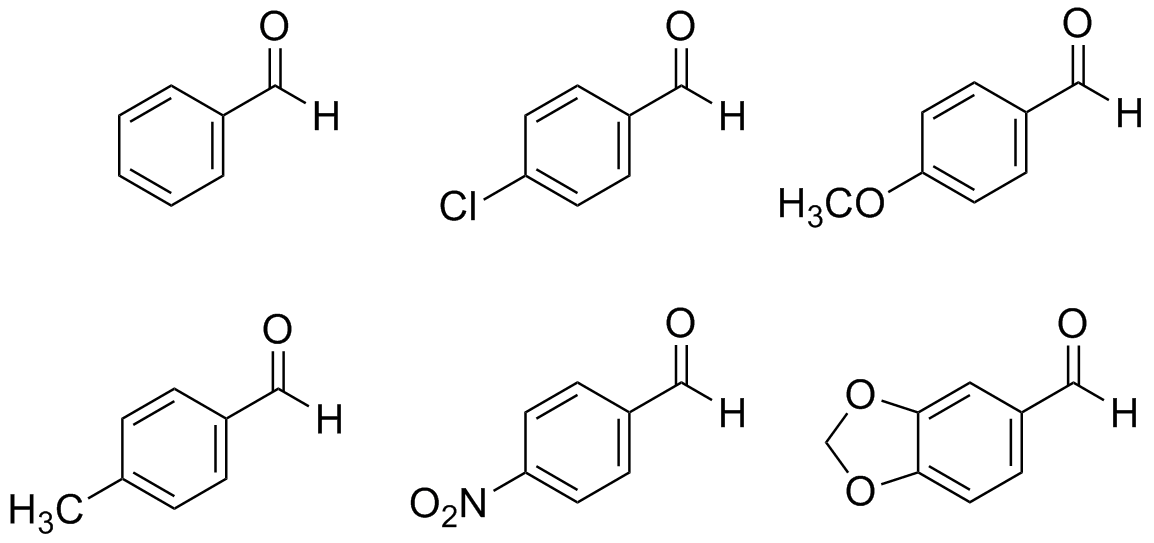
Choose one of the two focus questions below (see Ege 17.4 Section A (pp. 701-705). Note the outcome of aldol condensation reaction is significantly influenced by the electronics of both the aldehyde and ketone.
- Which two of the following aldehydes below are reactive in the aldol condensation reaction? Which two are least reactive in the aldol condensation reaction?
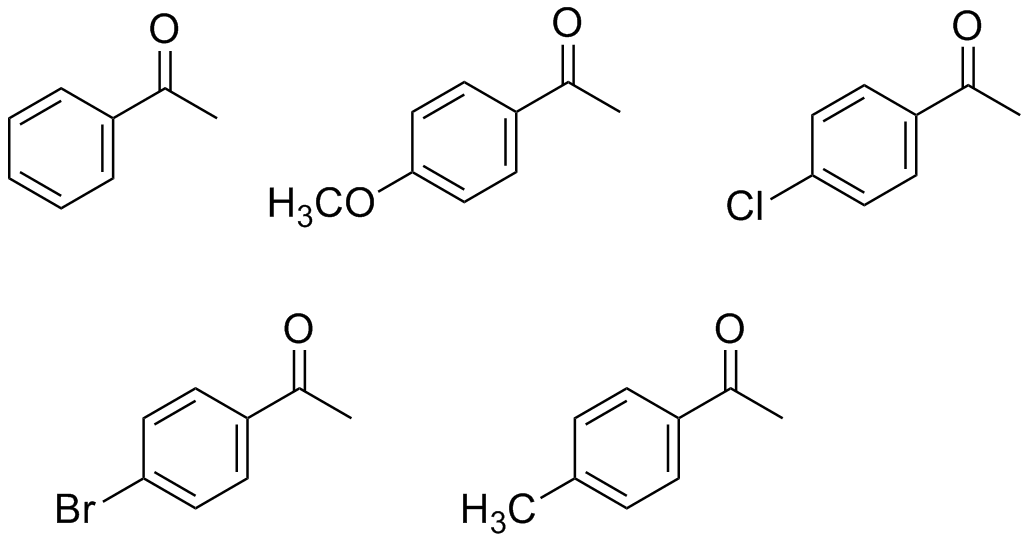
- Which two of the following ketones are most reactive in the aldol condensation reaction? Which two are least reactive in the aldol condensation reaction?
Creativity is encouraged. If you have a different idea for a focus question please discuss it with your GSI before proceeding.
Before lab discuss your focus question with your small group and answer the questions found on the group experimental design sheet at the link here. The sheet is also provided in the resources folder on CTools.
General Aldol Condensation Procedure
Place ~ 1 mmol (weigh accurately) of the aldehyde into a conical vial equipped with a magnetic spin vane. Add one mole equivalent amount of the ketone and 1 mL of 95 % ethanol to the vial and start stirring. Add 0.10 mL of a 15 M aqueous sodium hydroxide solution to the vial, cap, and stir at room temperature until it solidifies (CAUTION! NaOH is a strong base). Depending on how you’ve altered the reaction conditions the reaction may take more or less time.
Most of the chalcone products will precipitate out of solution after forming. Break up the solid with a spatula and dilute with 2 mL of ice water. Transfer the mixture into another 3 mL of ice water in a small Erlenmeyer flask. Stir thoroughly, then suction filter, wash with cold water, and allow to air dry before you determine the crude yield. All aldol condensation products should be purified by recrystallization, and most can be recrystallized from 95% ethanol.
Characterization
The purity of all products should be checked by TLC and m.p., and their IR spectrum recorded. See Table 4-1 for melting point data for possible Experiment 4 products. Note the m.p. data for some products is not available in the literature.
Post-lab Questions
Write your answer at the end of your lab notebook pages for this experiment
- What would you do differently if you could modify the design of your experiment?
- Was your hypothesis correct (it’s OK if it wasn’t)? What results either supported or refuted your hypothesis?
| R | R' | mp (°C) |
|---|---|---|
| H | 4'-OCH3 | 106 |
| H | 4'-Cl | 100 |
| H | 4'-Br | 104-105; 113 |
| H | 4'-CH3 | 59-60; 77-78 |
| 4-NO2 | H | 165 |
| 4-NO2 | 4’-OCH3 | 167-168 |
| 4-NO2 | 4’-Cl | 163-164 |
| 4-NO2 | 4’-Br | 166 |
| 4-NO2 | 4'-CH3 | 162 |
| 4-CH3 | H | 96.5 |
| 4-CH3 | 4’-OCH3 | ? |
| 4-CH3 | 4’-Cl | 165 |
| 4-CH3 | 4’-Br | ? |
| 4-CH3 | 4'-CH3 | 127.5 |
| 4-OCH3 | H | 77 |
| 4-OCH3 | 4’-OCH3 | 102 |
| 4-OCH3 | 4’-Cl | 121-122; 128 |
| 4-OCH3 | 4’-Br | 142-143 |
| 4-OCH3 | 4'-CH3 | 94 |
| 4-Cl | H | 103; 113-114 |
| 4-Cl | 4’-OCH3 | 130-131 |
| 4-Cl | 4’-Cl | 156-157 |
| 4-Cl | 4’-Br | ? |
| 4-Cl | 4'-CH3 | ? |
| 3,4-O-CH2-O | H | 122 |
| 3,4-O-CH2-O | 4’-OCH3 | 129 |
| 3,4-O-CH2-O | 4’-Cl | 128 |
| 3,4-O-CH2-O | 4’-Br | ? |
| 3,4-O-CH2-O | 4'-CH3 | 130 |


