VSEPR
- Page ID
- 25336
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Valence Shell Electron Pair Repulsion Model
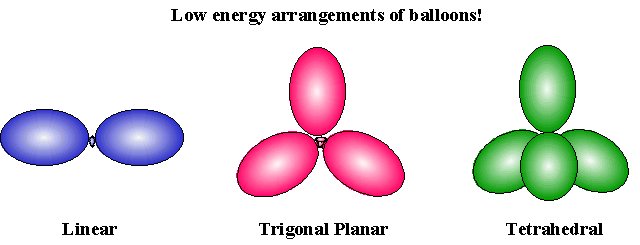
Balloons tied together adopt arrangements which minimize steric clashes between neighbors:
- Atoms are bonded together by electron pairs in valence orbitals
- Electrons are all negatively charged and tend to repel other electrons
- Bonding pairs of shared electrons tend to repel other bonding pairs of electrons in the valence orbital
The best spatial arrangement of the bonding pairs of electrons in the valence orbitals is one in which the repulsions are minimized
Like the balloon example:
- Two electron pairs in the valence orbital are arranged linearly
- Three electron pairs are organized in a trigonal planar arrangement
- Four electron pairs are organized in a tetrahedral arrangement
- Five electron pairs are arranged in a trigonal bipyramid
- Six electron pairs are organized in an octahedral arrangement
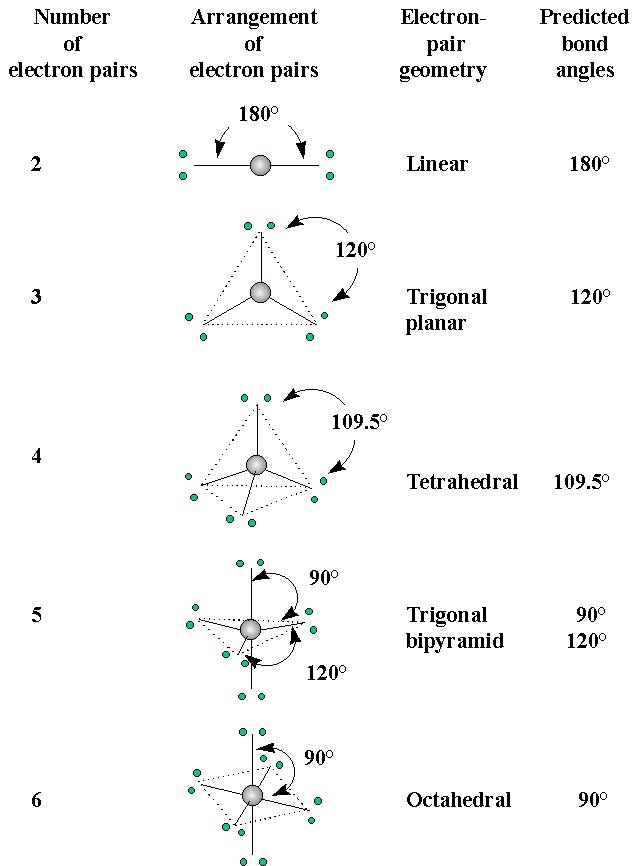
The shape of a molecule can be related to these five basic arrangements
Predicting Molecular Geometries
In Lewis structures there are two types of valence electron pairs:
- bonding pairs (shared by atoms in bonds)
- nonbonding pairs (also called lone pairs)
The Lewis structure of ammonia:
- Three bonding pairs of electrons
- One nonbonding pair of electrons
The electron shell repulsion between these four electron pairs is minimized in a tetrahedral arrangement (i.e. the "electron pair geometry" is tetrahedral)
This arrangement is for the valence electron pairs. What about the atoms in a compound?
- The molecular geometry is the location of the atoms of a compound in space
- We can predict the molecular geometry from the electron pair geometry
- In the above example (ammonia), we would predict that the three hydrogens would form the vertices of a tetrahedron, and the nonbonding electron pair the fourth. Thus, ammonia would have a trigonal pyramide arrangement of its H atoms
Steps involved in determining the VSEPR model:
- Draw the Lewis structure
- Count total number of electron pairs around the central atom. Arrange them to minimize the electron shell repulsion
- Describe the molecular geometry in terms of the angular arrangement of the bonding pairs
Four or Fewer Valence-Shell Electron Pairs
Structural types for molecules or ions which obey the octet rule:
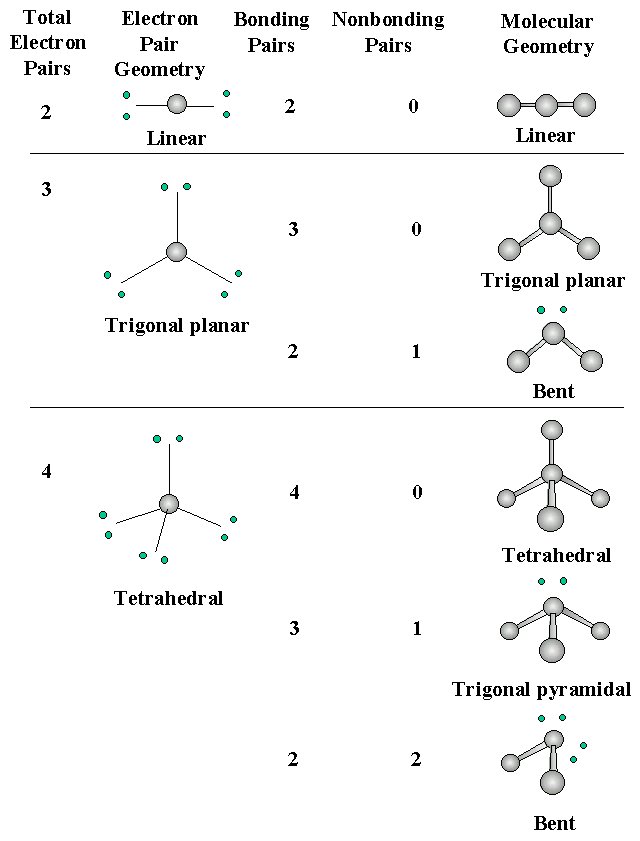
Note: a double or triple bond is counted as one bonding pair when predicting geometry
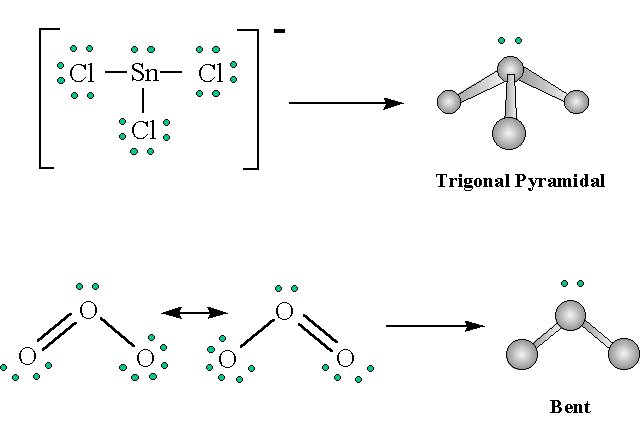
Using the VSEPR model predict the molecular geometries of a) SnCl3- and b) O3
The Effect of Nonbonding Electrons and Multiple Bonds on Bond Angles
The VSEPR model can be used to explain slight distortions from ideal bond geometries observed in some structures. Methane, ammonia and water all have tetrahedral electron-pair geometries, but the bond angles of ammonia and water are slightly distorted from an ideal tetrahedron:
The bond angles decrease as the number of nonbonding electron pairs increases
Since the electron pairs of bonding atoms are somewhat delocalized from the individual atoms (i.e. they are shared by two atoms), whereas the nonbonding electron pairs are attracted to a single nucleus, the nonbonding pairs can be thought of as having a somewhat larger electron cloud near the parent atom (kind of like being a somewhat larger balloon in the balloon analogy). This "crowds" the bonding pairs and the geometry distortions reflect this.
Multiple bonds, which contain higher electron density than single bonds also distort geometry by crowing the bonding pairs of single bonds:
Electrons in multiple bonds, like nonbonding electrons, exert a greater repulsive force on adjacent electron pairs than do single bonds
Geometries of Molecules with Expanded Valence Shells
When the central atom has 'd' orbitals available (n = 3 and higher) then it may have more than 4 electron pairs around it. Such atoms exhibit a variety of molecular geometries:
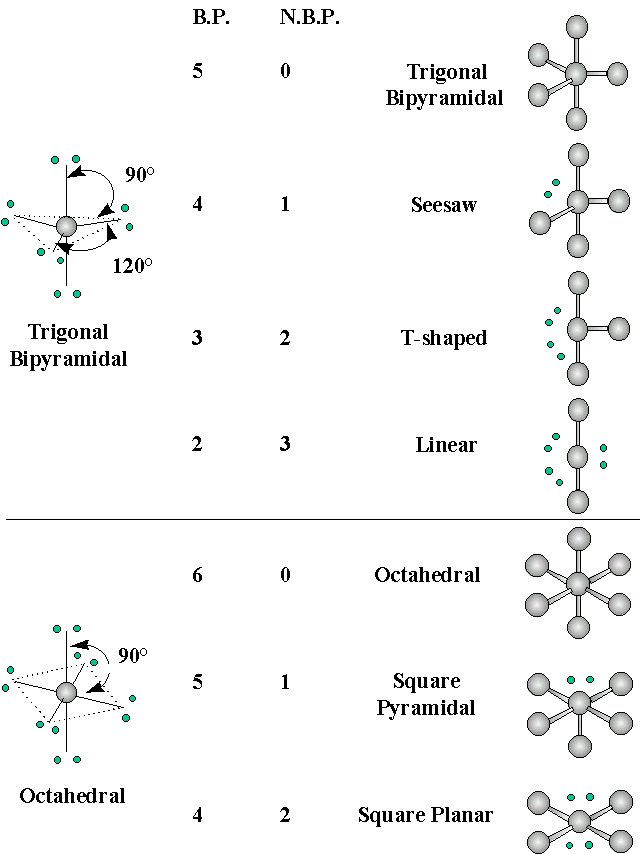
The trigonal bipyramidal arrangement for atoms with 5 pairs of valence electrons contains two geometrically distinct types of electron pairs, axial and equitorial:
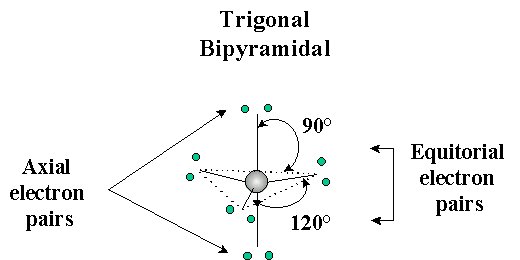
If there is a non-bonding pair of electrons (a "larger" electron cloud), it will go in the axial position to minimize electron repulsion
The octahedral structure contains 6 pairs of valence electrons. All positions are equivalent and at 90° from other electron pairs. If there is one nonbonding pair of electrons, it makes no difference where we place them. However, if there are two nonbonding pairs of electrons, the second pair will be 180° from the first to minimize steric interactions
Molecules with no central atom
The VSEPR model can be used to determine the geometry of more complex molecules
- The first carbon has four pairs of valence electrons and will be tetrahedral
- The second carbon has "three" (multiple bonds count as one in VSEPR) and will be trigonal planar
- The oxygen on the right has four and will be tetrahedral (only two have bonds and thus it will appear as a "bent" conformation):
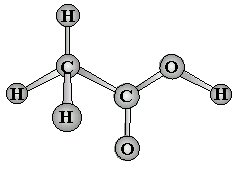
Examples of molecular geometry:









