9.5: Oxidative Phosphorylation
- Page ID
- 434000
- Understand parts 2 and 3 of the third stage of food catabolism, i.e., oxidative phosphorylation, and the basics of the redox chemistry of the molecules involved, including flavin mononucleotide, ubiquinone, and cytochrome c.
- Understand the electron transport process basics, including basic redox reactions and proton pumping in complexes I through IV.
- Understand the ATP synthesis process, accounting for the ATP yield, body heating, and reactive oxygen species produced.
What is oxidative phosphorylation?
In food catabolism up to this point, the energy is transferred from food to high-energy molecules, such as energetic electron carriers \(\ce{NADH}\) and \(\ce{FADH2}\). Recall that these electron carrier molecules do not deliver elections alone; they deliver protons and electrons, i.e., \(\ce{2H^{+} + 2e^{-}}\). The electrons in \(\ce{NADH}\) and \(\ce{FADH}\) are transported through a series of enzymes located in the inner membrane of mitochondria, and ultimately, the \(\ce{2H^{+} + 2e^{-}}\) reduce \(\ce{O}\) into \(\ce{H2O}\) by the reaction shown below.
\(\ce{4H^{+} + 4e^{-} + O2 -> 2H2O}\)
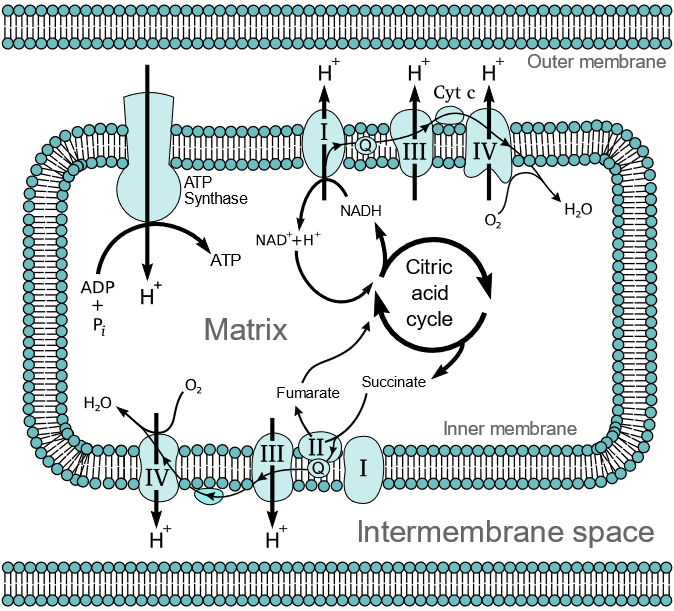
In each step, the electrons are transferred from a substance with a higher reduction potential to one with a lower reduction potential. Energy is released in each step proportional to the difference in the reduction potential. The energy is converted to electrochemical potential energy by pumping protons (\(\ce{H^{+}}\)) from the matrix to intermembrane spaces through sets of enzymes called complexes I, III, & IV. The electrochemical energy is harnessed by \(\ce{ATP}\) synthase, which allows protons to flow back from the intermembrane space to the matrix and couples the energy released to synthesize higher energy \(\ce{ATP}\) by phosphorylating lower energy \(\ce{ADP}\) with inorganic phosphate (\(\ce{P_i}\)). These interconnected processes of electron transport and \(\ce{ATP}\) synthesis are collectively called oxidative phosphorylation. It happens in the mitochondria of cells as illustrated in Figure \(\PageIndex{1}\) and is explained in the following sections.
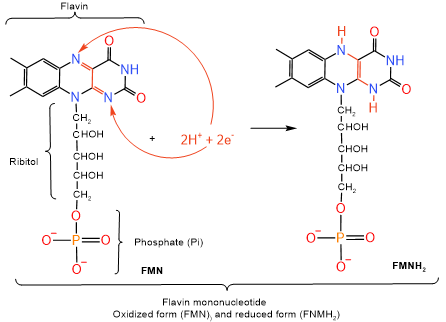
 In addition to \(\ce{NAD^{+}}\) and \(\ce{FAD}\), the following molecules are involved in the transport of electrons and protons in the electron transport chain. The first electron acceptor from \(\ce{NADH}\) or \(\ce{FADH2}\) in the electron transport chain is flavin mononucleotide which changes from its oxidized form represented as \(\ce{FMN}\) to its reduced form represented as \(\ce{FMNH2}\) as illustrated in the figure on the right. Structure of \(\ce{FMN}\) comprises three parts, phosphate, ribitol, and flavin. It is the same as one of the two nucleotides in flavin adenine dinucleotide. Nitrogen atoms in flavin part of \(\ce{FMN}\) take part in the redo process, as illustrated in the figure on the right. \(\ce{FMNH2}\) pass on the electrons to iron-sulfur clusters, which include \(\ce{[2Fe-2S]}\) and \(\ce{[4Fe-4S]}\) clusters. The redox couple in iron-sulfur clusters is \(\ce{Fe^{3+}/Fe^{2+}}\), i.e., the following redox reaction:
In addition to \(\ce{NAD^{+}}\) and \(\ce{FAD}\), the following molecules are involved in the transport of electrons and protons in the electron transport chain. The first electron acceptor from \(\ce{NADH}\) or \(\ce{FADH2}\) in the electron transport chain is flavin mononucleotide which changes from its oxidized form represented as \(\ce{FMN}\) to its reduced form represented as \(\ce{FMNH2}\) as illustrated in the figure on the right. Structure of \(\ce{FMN}\) comprises three parts, phosphate, ribitol, and flavin. It is the same as one of the two nucleotides in flavin adenine dinucleotide. Nitrogen atoms in flavin part of \(\ce{FMN}\) take part in the redo process, as illustrated in the figure on the right. \(\ce{FMNH2}\) pass on the electrons to iron-sulfur clusters, which include \(\ce{[2Fe-2S]}\) and \(\ce{[4Fe-4S]}\) clusters. The redox couple in iron-sulfur clusters is \(\ce{Fe^{3+}/Fe^{2+}}\), i.e., the following redox reaction:
\(\ce{Fe^{3+} + e^{-} <=>[Reduction][Oxidation] Fe^{2+}}\).
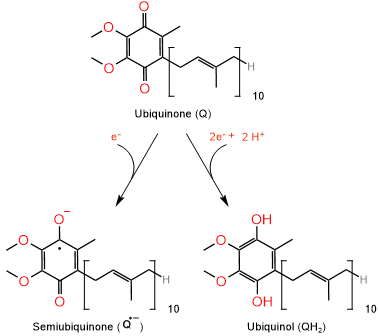
 Finally, the electrons are transferred to coenzyme-Q, which in oxidized form is ubiquinone represented as \(\ce{Q}\) and in its reduced form is ubiquinol represented as \(\ce{QH2}\), as illustrated in the figure above on the left. Coenzyme-Q is hydrophobic, i.e., lipid-soluble. Coenzyme-Q moves freely in the hydrophobic environment within the biliary inner membrane to pass on electrons from complex I and II to complex III. Coenzyme-Q not only can accept two electrons and two protons, but it can also accept one electron to become semiubiquinone, a resonance-stabilized radical anion.
Finally, the electrons are transferred to coenzyme-Q, which in oxidized form is ubiquinone represented as \(\ce{Q}\) and in its reduced form is ubiquinol represented as \(\ce{QH2}\), as illustrated in the figure above on the left. Coenzyme-Q is hydrophobic, i.e., lipid-soluble. Coenzyme-Q moves freely in the hydrophobic environment within the biliary inner membrane to pass on electrons from complex I and II to complex III. Coenzyme-Q not only can accept two electrons and two protons, but it can also accept one electron to become semiubiquinone, a resonance-stabilized radical anion.
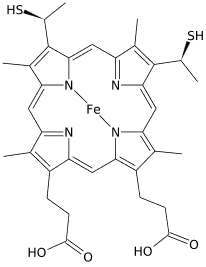
Cytochrome c (Cyt c) is another electron carrier protein with a heme group attached. Heme group is illustrated in the figure on the right. Iron in the heme group of cytochrome c is the redox couple (\(\ce{Fe^{3+}/Fe^{2+}}\)) as in the case of iron-sulfur clusters. \(\ce{Fe^{3+}}\) changes to \(\ce{Fe^{2+}}\) when an electron is added to it, and the reverse happens when an electron is removed. Cytochrome c is water soluble and travels between complex III and complex IV in the watery environment along the inner membrane's surface facing intermembrane space.
Electron transport chain
In this part 2 of stage 3, electrons are removed from \(\ce{NADH}\) and \(\ce{FADH2}\) and passed on to reduce \(\ce{O2}\) into \(\ce{H2O}\) through a series of enzyme complexes, i.e., complexes I through IV and mobile electron carriers like ubiquinone (\(\ce{Q}\)) and cytochrome c (\(\ce{Cyt \; c}\)) by a process called electron transport chain. At each step, electrons pass from a substance with a higher reduction potential to one with a lower one. Energy is released proportional to the difference in the reduction potentials.
The complex I, III, and IV extend from the matrix to the intermembrane space through the inner membrane, as illustrated in Figure \(\PageIndex{1}\). These three complexes utilize the energy released to pump protons (\(\ce{H^{+}}\)) from the matrix to the intermembrane space. Complex II extends to the matrix from the inner membrane but does not cross the membrane to intermembrane space. Therefore complex II transports electrons but does not pump protons. Coenzyme-Q (\(\ce{Q}\)) is a hydrophobic electron carrier molecule that moves in a hydrophobic environment inside the lipid bilayer of the inner membrane from complexes I and II to complex III. Cytochrome c (\(\ce{Cyt \; c}\)) is a hydrophilic electron carrier molecule that moves along the surface of the inner membrane facing the intermembrane space from complex III to complex IV. The enzyme complexes and the mobile electron carriers involved in the electron transport chain and \(\ce{ATP}\) synthesis are described in the following sub-sections, where the chemical equations and figures are taken from the public domain source via Wikipedia.
Complex I
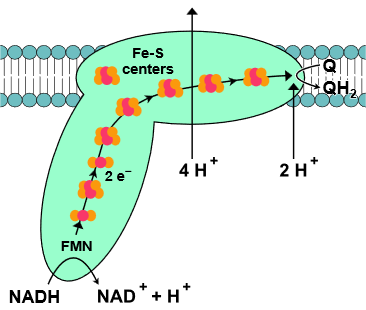 Complex I, also known as NADH-coenzyme Q oxidoreductase or NADH dehydrogenase, is the first enzyme complex in the electron transport chain. Complex I is illustrated in the figure on the right. The electron transport begins when an NADH binds with and donates two electrons to complex I. The electrons are received by flavin mononucleotide (\(\ce{FMN}\)) attached to the complex, which is reduced to \(\ce{FMNH2}\) form. Then the electrons are transferred through a series of iron-sulfur clusters within complex I to coenzyme-Q (\(\ce{Q}\)) that, in turn, reduces to its reduced form \(\ce{QH2}\). \(\ce{QH2}\) travels through the lipid bilayer to and delivers the electrons to complex III.
Complex I, also known as NADH-coenzyme Q oxidoreductase or NADH dehydrogenase, is the first enzyme complex in the electron transport chain. Complex I is illustrated in the figure on the right. The electron transport begins when an NADH binds with and donates two electrons to complex I. The electrons are received by flavin mononucleotide (\(\ce{FMN}\)) attached to the complex, which is reduced to \(\ce{FMNH2}\) form. Then the electrons are transferred through a series of iron-sulfur clusters within complex I to coenzyme-Q (\(\ce{Q}\)) that, in turn, reduces to its reduced form \(\ce{QH2}\). \(\ce{QH2}\) travels through the lipid bilayer to and delivers the electrons to complex III.
As the electrons move through complex I and release part of their energy, four protons (\(\ce{4H^{+}}\)) are pumped from the matrix to intermembrane space by utilizing the energy released. It generated polarity across the inner membrane, with the positive side (P-side) facing the intermembrane area and the negative side (N-side) facing the matrix. The exact mechanics of proton pumping is not yet clearly understood. Still, the research indicates that it happens through conformational changes in complex I such that the protein bind protons on the N-side and releases them on the P-side of the membrane. The overall redox reaction in complex I is the following.
\(\ce{NADH + Q +5H^{+}_{matrix} -> NAD^{+} + QH_{2} + 4H^{+}_{intermembrane}}\)
\(\ce{NAD^{+}}\) liberated in this reaction becomes available to oxidize more substrates in the metabolic pathways.
Complex II
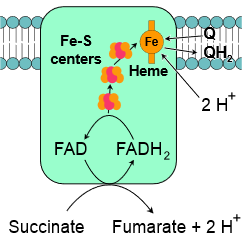 Complex II, also known as succinate-Q oxidoreductase or succinate dehydrogenase, is a second entry point to the electron transport chain. It is part of the citric acid cycle and the electron transport chain. It contains protein subunits bonded with flavin adenine dinucleotide \(\ce{FAD}\), iron-sulfur clusters, and a heme group, as illustrated in the figure on the right. Succinate is oxidized to fumarate in the citric acid cycle at the expense of oxidation of \(\ce{FAD}\) to \(\ce{FADH2}\) in complex II. The electrons and protons transfer from \(\ce{FADH2}\) through iron-sulfur clusters to coenzyme-Q in complex II by the following overall reaction.
Complex II, also known as succinate-Q oxidoreductase or succinate dehydrogenase, is a second entry point to the electron transport chain. It is part of the citric acid cycle and the electron transport chain. It contains protein subunits bonded with flavin adenine dinucleotide \(\ce{FAD}\), iron-sulfur clusters, and a heme group, as illustrated in the figure on the right. Succinate is oxidized to fumarate in the citric acid cycle at the expense of oxidation of \(\ce{FAD}\) to \(\ce{FADH2}\) in complex II. The electrons and protons transfer from \(\ce{FADH2}\) through iron-sulfur clusters to coenzyme-Q in complex II by the following overall reaction.
\(\ce{Succinate + Q -> Fumarate + QH2}\)
Coenzyme-Q passes on the electrons from complex II to complex III as from complex I to complex III.
Recall that (\ce{FADH2}\) is less energetic than (\ce{NADH}\). Since oxidation \(\ce{FADH2}\) to \(\ce{FAD}\) at complex II releases less energy than oxidation of \(\ce{NADH}\) to \(\ce{NAD^{+}}\) at complex I, complex II do not pump protons from matrix to intermembrane space. The energy released by electron transport is utilized to pump protons in complex III and IV, as described in the next section.
Complex III
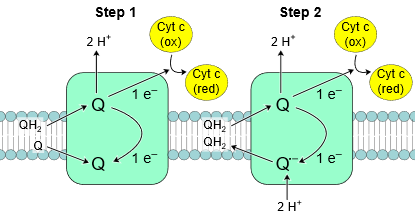 Complex III, also known as cytochrome C reductase or cytochrome bc1 complex, contains protein subunits, an iron-sulfur cluster, and cytochromes. A cytochrome is a protein with at least one heme group that transfers one electron by accepting and releasing an electron based on the following reaction:
Complex III, also known as cytochrome C reductase or cytochrome bc1 complex, contains protein subunits, an iron-sulfur cluster, and cytochromes. A cytochrome is a protein with at least one heme group that transfers one electron by accepting and releasing an electron based on the following reaction:
\(\ce{Fe^{3+} + e^{-} <=> Fe^{2+}}\)
Complex III passes on electrons from coenzyme-Q (\(\ce{QH2}\)) to cytochrome c (\(\ce{Cyt~ c}\)). Since \(\ce{QH2}\) delivers two electrons (\(\ce{e^{-}}\)) while \(\ce{Cyt~ c}\) can accept only one, the process happens in two steps, as illustrated in the figure on the right. In the first step, \(\ce{QH2}\) transfers one \(\ce{e^{-}}\) to \(\ce{Cyt~ c}\) and one to another coenzyme-Q in quinone (\(\ce{Q}\)) form and releases \(\ce{2H^{+}}\). \(\ce{Q}\) converts to a semiubiquinone (\(\ce{Q^{\bullet -}}\)). In the second step, another \(\ce{QH2}\) transfers one electron to a second \(\ce{Cyt~ c}\) and one to \(\ce{Q^{\bullet -}}\) converting it to \(\ce{Q}\) which is released from the complex and repeats the above two steps. This way, two electrons are transferred from a \(\ce{Q}\) to two \(\ce{Cyt~ c}\) and \(\ce{4H^{+}}\) are pumped out into intermembrane space by complex III as shown in the following reaction.
\(\ce{QH2 + 2Cyt \; c_{ox} + 2H^{+}_{matrix} -> Q + 2Cyt \; c_{red} + 4H^{+}_{intermembrane}}\)
\(\ce{Cyt~ c}\) carries the electron to complex IV.
Complex IV
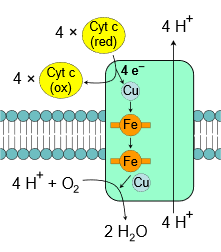 Complex IV, also known as cytochrome c oxidase, is the final complex in the electron transport chain. It contains several subunits, two heme groups, and several metal ion cofactors, including three atoms of copper, one magnesium, and one zinc. Complex IV reduces oxygen into water at the expense of oxidation of \(\ce{Cyt~c}\) and pumps \(\ce{4H^{+}}\) from matrix to intermembrane space as illustrated in the figure on the right and shown in the following overall reaction.
Complex IV, also known as cytochrome c oxidase, is the final complex in the electron transport chain. It contains several subunits, two heme groups, and several metal ion cofactors, including three atoms of copper, one magnesium, and one zinc. Complex IV reduces oxygen into water at the expense of oxidation of \(\ce{Cyt~c}\) and pumps \(\ce{4H^{+}}\) from matrix to intermembrane space as illustrated in the figure on the right and shown in the following overall reaction.
\(\ce{4Cyt \; c_{red} + O2 + 8H^{+}_{matrix} -> 4Cyt \; c_{ox} + 2H2O + 4H^{+}_{intermembrane}}\)
\(\ce{ATP}\)-synthase
\(\ce{ATP}\)-synthase, also known as complex V is the final complex in the oxidative phosphorylation process, i.e., part 3 of stage 3 of food catabolism. It comprises two parts in the 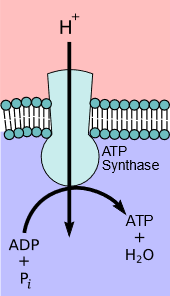 shape of a mushroom, as illustrated in the figure on the left. The first part, FO, is the hydrophobic part embedded in the inner membrane (a blue
shape of a mushroom, as illustrated in the figure on the left. The first part, FO, is the hydrophobic part embedded in the inner membrane (a blue 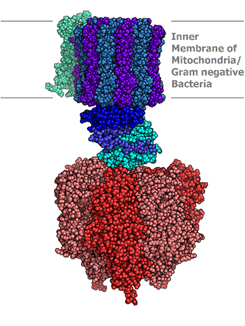 and purple region in the model image on the right (Copyright; Alex. X, CC BY-SA 3.0, via Wikimedia Commons). The FO acts as a pore for the flow of protons (\(\ce{H^{+}}\)) across the membrane. The second part is F1 which is hydrophilic and spheroidal and protrudes into the matrix (red area in the image on the right). \(\ce{ATP}\) synthesis takes place in the F1 part of \(\ce{ATP}\)-synthase. It operates on the principle of chemiosmosis, which is described below.
and purple region in the model image on the right (Copyright; Alex. X, CC BY-SA 3.0, via Wikimedia Commons). The FO acts as a pore for the flow of protons (\(\ce{H^{+}}\)) across the membrane. The second part is F1 which is hydrophilic and spheroidal and protrudes into the matrix (red area in the image on the right). \(\ce{ATP}\) synthesis takes place in the F1 part of \(\ce{ATP}\)-synthase. It operates on the principle of chemiosmosis, which is described below.
Chemiosmosis
Water movement from the direction of higher to lower concentration across a semipermeable membrane is called osmosis. When protons (\(\ce{H^{+}}\)) are pumped from the matrix to intermembrane space by complex I, III, & IV, a proton concentration gradient is developed across the membrane. Since protons are positive charge ions (\(\ce{H^{+}}\)), there is also an electrical gradient; collectively, it is called an electrochemical gradient. Chemiosmosis is the flow of ions (\(\ce{H^{+}}\)) across a semipermeable membrane down their electrochemical gradient.
An electrochemical gradient developed due to protons (\(\ce{H^{+}}\)) is a form of electrochemical potential energy. \(\ce{ATP}\)-Synthase harnesses the electrochemical potential energy to synthesize \(\ce{ATP}\) as the protons flow through it, just as electricity is generated as water from a dam flows through a turbine. The higher energy \(\ce{ATP}\) is synthesized by condensing lower energy \(\ce{ADP}\) and phosphate \(\ce{P_{i}}\) in a process called oxidative phosphorylation. It takes three to four \(\ce{H^{+}}\) to synthesize one \(\ce{ATP}\) by the following overall reaction.
\(\ce{ADP + P_{i} +4H^{+}_{intermembrane} <=> ATP + H2O + 4H^{+}_{matrix}}\)
This is an equilibrium reaction, i.e., when the electrochemical gradient is low, \(\ce{ATP}\)-synthase consumes \(\ce{ATP}\) and returns protons from the matrix to the intermembrane space.
Structure and mechanisms of operation of \(\ce{ATP}\)-synthase
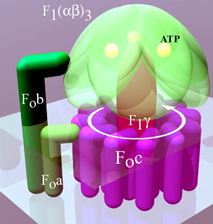
\(\ce{ATP}\)-synthase comprises several protein sub-units. It is in the shape of a mushroom with two major parts: a stem-like part which is a hydrophobic portion embedded in the inner membrane, is called FO, and a mushroom head-like part that protrudes into the matrix, is called F1, as illustrated in Figure \(\PageIndex{2}\) a. Note: the subscript in FO is the letter O, not the number zero.
 a
a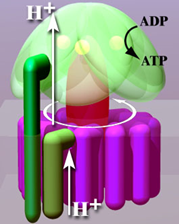 b
b c
c d
dFO part consists of six c subunits (FOc) arranged in a ring shape with proton channels between them that make the rotor part of this molecular machinery, as illustrated in Figure \(\PageIndex{2}\) a & b. The b subunit (FOb) connects to the F1 portion and prevents it from rotating. The subunit FOa connects FOb to the FOc ring. FOc part is embedded in the membrane and couples the energy released as the protons flow through the channels to kinetic energy in the form of rotation of the FOc ring, like a turbine moves by water in a hydroelectric dam. Other subunits are not described here.
F1 portion has a set of three \(\alpha\) and thee \(\beta\) subunits arranged alternately like carpels of an orange (F1(\(\alpha\)\(\beta\))3) with F1\(\gamma\) in the middle. The F1\(\gamma\) is like an axil connected with the rotary FOc, as shown in Figure \(\PageIndex{2}\) a. The F1(\(\alpha\)\(\beta\))3 is prevented from rotating by the FOb subunit, but F1\(\gamma\) rotates along with the rotor FOc ring. The rotation of F1\(\gamma\) within F1(\(\alpha\)\(\beta\))3 causes conformation changes in the \(\beta\) subunits, as illustrated in the video that will play by clicking this link. The conformational changes in the \(\beta\) subunits are linked to the mechanism of \(\ce{ATP}\) synthesis. Other subunits of the F1 portion are not described here.
The steps of the rotation of the
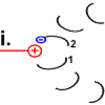
Steps in the rotation mechanism of Asw-hamburg at German Wikipedia., CC BY-SA 3.0, via Wikimedia Commons)
one (labeled #1 in step a), which, being near a cation group (\(\ce{peptide-NH3^{+}}\) of arg residue of
- Step c: The strained structure relaxes as the electrostatic tension is removed, causing the peptide to rotate.
- Step d: Twisting motion bring #1 back to the other side of the stator \(\ce{peptide-NH3^{+}}\) group causing the rotor .
- Step e: #1 subunit is neutral like other
- rotor #2 is under the spell of the positive charge of \(\ce{peptide-NH3^{+}}\) group.
- Step g: The #2 ionizes the \(\ce{-COOH) (
-
- rotation of the rotor, and
- one
The simulation of the movement described above is illustrated in Figure \(\PageIndex{2}\) c.
The F1(\(\alpha\)\(\beta\))3 complex catalyzes \(\alpha\)\(\beta\) dimers in the shape of carpels in an orange. It is the \(\beta\) subunits that catalyze the F1\(\gamma\) subunit inside the F1(\(\alpha\)\(\beta\))3 complex causes conformational changes in the F1\(\beta\) subunits that are linked with the mechanism of
F1\(\beta\) switched between three states with each 360o rotation of F1\(\gamma\) subunit inside the F1(\(\alpha\)\(\beta\))3 complex, as illustrated in Figure \(\PageIndex{2}\) d.
- First is the open-state, shown in brown, where
- F1\(\beta\) closes up around the substrate
- F1\(\beta\) tightens around the substrate forcing them to condense into an
- F1\(\beta\) reverts to an open-state that releases the
Since one \(\ce{H^{+}}\) cause a turn, a complete () turn of F1\(\gamma\) subunit inside F1(\(\alpha\)\(\beta\))3 complex needs \(\ce{12H^{+}}\) and produces three F1\(\beta\) subunits in it, that gives the following overall reaction.
\(\ce{ADP + P_{i} + 4H^{+}_{intermembrane} <=> ATP + H2O + 4H^{+}_{matrix}}\)
The next video presents the oxidative phosphorylation of glucose in a summary form.
\(\ce{NADH}\) is reduced in the electron transport chain at the expense of reduction of \(\ce{O2}\) by the following overall reaction.
\(\ce{1/2O2 + NADH + H^{+} -> H2O + NAD^{+}}\)
The potential difference between these redox pairs is 1.14 volts, equivalent to -218 kJ/mol. Reduction of one \(\ce{NADH}\) can produce three \(\ce{ATP}\). Production of \(\ce{ATP}\) costs 30.5 kJ/mole, which is equivalent to 30.5 kJ/mole x 3 = 91.5 kJ/mole of \(\ce{NADH}\). So the percentage of energy conserved as \(\ce{ATP}\), i.e., the energy efficiency is: \(\frac{91.5}{218}\times{100} = 42\text{%}\). The remaining 58% of energy ends up heating the body.
 Some compounds, known as uncouplers, uncouple electron transport from \(\ce{ATP}\) synthase, i.e., the electron transport pumps \(\ce{H^{+}}\) as usual but \(\ce{H^{+}}\) return to the matrix without producing \(\ce{ATP's}\). So, all energy released during the electron transport process becomes heat energy. Examples of uncouplers are 2,4-dinitrophenol and dicumarol that combine with \(\ce{H^{+}}\) and, being hydrophobic, carry them through the inner membrane. Some compounds, like oligomycin A prevent \(\ce{ATP}\) synthesis by blocking \(\ce{H^{+}}\)-channels in \(\ce{ATP}\) synthase. Salicylic acid (aspirin), if taken in extreme excess, also blocks \(\ce{H^{+}}\)-channels in \(\ce{ATP}\) synthase. When the electron transport chain operates without \(\ce{ATP}\) synthesis, all of the electron transport energy is used as heat.
Some compounds, known as uncouplers, uncouple electron transport from \(\ce{ATP}\) synthase, i.e., the electron transport pumps \(\ce{H^{+}}\) as usual but \(\ce{H^{+}}\) return to the matrix without producing \(\ce{ATP's}\). So, all energy released during the electron transport process becomes heat energy. Examples of uncouplers are 2,4-dinitrophenol and dicumarol that combine with \(\ce{H^{+}}\) and, being hydrophobic, carry them through the inner membrane. Some compounds, like oligomycin A prevent \(\ce{ATP}\) synthesis by blocking \(\ce{H^{+}}\)-channels in \(\ce{ATP}\) synthase. Salicylic acid (aspirin), if taken in extreme excess, also blocks \(\ce{H^{+}}\)-channels in \(\ce{ATP}\) synthase. When the electron transport chain operates without \(\ce{ATP}\) synthesis, all of the electron transport energy is used as heat.
Certain animals adapted to the cold environment have developed uncoupling systems to generate more heat for heating their body. They contain a large amount of brown fat, a tissue with many mitochondria, and are brown due to iron in the cytochrome in the mitochondria. The electron transport works in the brown fat. Still, the mitochondria have certain proteins embedded in the inner membrane that allow \(\ce{H^{+}}\) to return to the matrix without \(\ce{ATP}\) production. The body of newborn babies loses more heat per unit mass because of the larger surface area to mass ratio. Newborn babies have brown fat deposits on arteries that heat the blood circulating in their bodies. Adults usually do not have brown fat except those who live and work in a cold climate.
 2,4-Dinitrophoenol
2,4-Dinitrophoenol Dicumarol
Dicumarol Oligomycin A
Oligomycin A Salicylic acid
Salicylic acidThe electron transport chain uses four electrons and four protons to reduce oxygen by the following overall reaction,
\(\ce{O2 + 4e^{-} + 4H^{+} -> 2H2O}\).
However, some electrons, particularly during the reduction of coenzyme-\(\ce{Q}\) in the complex-III, leak and cause the following reaction.
 \(\ce{O2 ->[e^{-}] \underset{Superoxide}{O2^{\bullet-}} ->[e^{-}] \underset{Peroxide}{O2^{2-}}}\)
\(\ce{O2 ->[e^{-}] \underset{Superoxide}{O2^{\bullet-}} ->[e^{-}] \underset{Peroxide}{O2^{2-}}}\)
Species like superoxide (\(\ce{O2^{\bullet{-}}}\)), peroxide (\(\ce{O2^{2-}}\)) and their product hydroxyl radical (\(\ce{HO^{\bullet}}\)) are called reactive oxygen species which are harmful. They damage proteins, cause mutations by reacting with DNA, and are responsible for aging, as illustrated in the figure on the right (taken from https://www.hiclipart.com/free-trans...-clipart-npvtr). The body has mechanisms to suppress the production of reactive species and destroy them if formed. Production of reactive oxygen species increases with an increase in the inter-member potential. The reactive oxygen species, oxidants, activate uncoupling proteins that reduce the membrane potential. Some substances in the body, e.g., vitamins C, E and antioxidant enzymes, react with and destroy the reactive oxygen species.
Summary of glucose catabolism
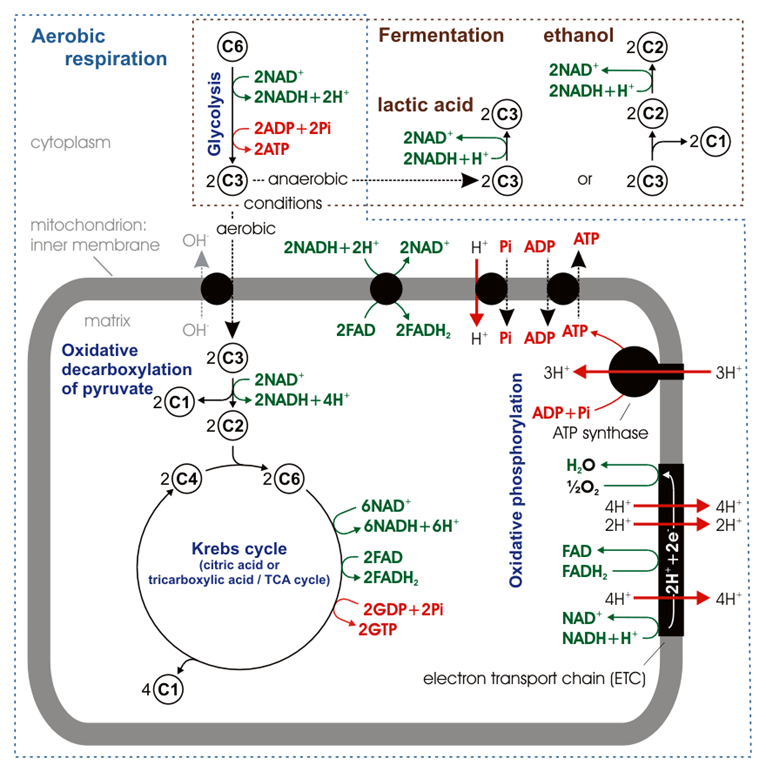
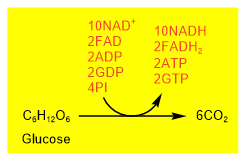 Aerobic catabolism of a glucose molecule converts a \(\ce{6C's}\) glucose molecule to six carbon dioxide along with \(\ce{ATP's}\), \(\ce{GTP's}\), and reduced coenzyme \(\ce{NADH's}\), and \(\ce{FADH2{'s}}\), as shown in the figure on the right.
Aerobic catabolism of a glucose molecule converts a \(\ce{6C's}\) glucose molecule to six carbon dioxide along with \(\ce{ATP's}\), \(\ce{GTP's}\), and reduced coenzyme \(\ce{NADH's}\), and \(\ce{FADH2{'s}}\), as shown in the figure on the right.
Glucose first goes through glycolysis that produces two pyruvate and overall two \(\ce{ATP}\) and two \(\ce{NADH}\). Two pyruvates go through oxidative decarboxylation producing two acetyl-\(\ce{CoA}\) and two \(\ce{NADH}\). Each acetyl-\(\ce{CoA}\) goes through one turn of the citric acid cycle. So, the two turns of the citric acid cycles convert two acetyl-\(\ce{CoA}\) into four \(\ce{CO2}\), six \(\ce{NADH}\), two \(\ce{FADH2}\), and two \(\ce{GDP}\). These steps are summarized in Figure \(\PageIndex{3}\) and Figure \(\PageIndex{4}\) shown below.
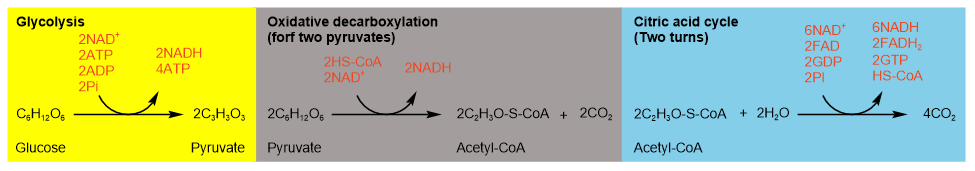
Figure \(\PageIndex{3}\) illustrates the summary of aerobic catabolism of glucose and production of \(\ce{ATP's}\), \(\ce{GTP's}\), and reduced coenzyme \(\ce{NADH's}\), and \(\ce{FADH2{'s}}\) at different stages.
Insulin is a hormone that regulates blood glucose levels. The glucose level increases after a meal, and the insulin promotes its absorption into the liver, fat, and muscle cells, converting it into glycogen or fats. High levels of insulin also inhibit the production of glucose by the liver. Low insulin levels have the opposite effects, which happen when a person has diabetes.
Comparison of \(\ce{ATP}\) yield in aerobic and anaerobic catabolism of glucose
Anaerobic catabolism, i.e., the two forms of fermentation shown in Figure \(\PageIndex{3}\) yields a net two \(\ce{ATP's}\) from a glucose molecule.
Aerobic catabolism yields about thirty \(\ce{ATP's}\). Two \(\ce{ATP's}\) are produced before the citric acid cycle and two \(\ce{GTP's}\), ten \(\ce{NADH's}\), and two \(\ce{FADH2{'s}}\) are produced during citric acid cycle. \(\ce{GTP's}\) later convert to \(\ce{ATP's}\). the reduced coenzymes, i.e., \(\ce{NADH's}\) and \(\ce{FADH2{'s}}\) enter the electron transport chain and produce heat and \(\ce{ATP's}\) as explained in the next section.
Two \(\ce{ATP's}\) are produced before the citric acid cycle along with four \(\ce{NADH}\). The citric acid cycle yields \(\ce{GTP's}\), six \(\ce{NADH's}\), and two \(\ce{FADH2{'s}}\). \(\ce{GTP's}\) convert to \(\ce{ATP's}\), i.e., one \(\ce{GTP}\) is equivalent to one \(\ce{ATP}\). \(\ce{NADH's}\) and \(\ce{FADH2{'s}}\) enter the electron transport chain and produce heat and \(\ce{ATP's}\). Recall that \(\ce{NADH}\) is more energetic and pumps more proton through complex I, III, and IV than \(\ce{FADH2}\) which is less energetic and pumps less protons through complex III and IV only. Therefore, \(\ce{NADH}\) yields more \(\ce{ATP's}\) than \(\ce{FADH2}\). Earlier books state that one \(\ce{NADH's}\) is equivalent to three \(\ce{ATP's}\), but currently accepted average values are the following.
\(\ce{GTP}~ \approx~ 1\ce{ATP}\)
\(\ce{NADH} ~\approx~ 2.5\ce{ATP}\)
\(\ce{FADH2} ~\approx~ 1.5\ce{ATP}\)
Based on these conversions, complete aerobic respiration of glucose produces approximately \(\ce{32ATP's}\), as shown in the following equation and detailed in Table 1.
\(\ce{\underbrace{C6H12O6}_{Glucose} + 6O2 + 10NAD^{+} + 2FAD + + 2GTP + 2ADP + 4Pi -> 6CO2 + 6H2O + 10NADH + 2FADH2 + 2GTP + 2ATP}\)
\(\ce{\underbrace{C6H12O6}_{Glucose} + 6O2 +32ADP + 32Pi -> 6CO2 + 6H2O + 32ATP}\)
| Reaction | Total | |
|---|---|---|
| Glycolysis | ||
| glucose → glucose-6-phosphate | -1 | |
| fructose 6-phosphate → fructose 1,6-bisphosphate | -1 | |
| 2 glyceraldehyde-3-phosphate → 2 1,3-bisphosphoglycerat | 5 | |
| 2 1,3-bisphosphoglycerate → 2 3-phosphoglycerate | 2 | |
| 2 phosphoenolpyruvate → 2 pyruvate | 2 | |
| Oxidative phosphorylation of two pyruvate | ||
| 2 pyruvate → 2 acetyl-CoA | 5 | |
| Two turns citric acid cycles to consume to 2 acetyl-\(\ce{CoA}\) | ||
| 2 isocitrate → 2 \(\alpha\)-ketoglutarate | 5 | |
| 2 α-ketoglutarate → 2 succinyl-\(\ce{CoA}\) | 5 | |
| 2 succinyl-CoA → 2 succinate | 2 | |
| 2 succinate → 2 fumarate | 3 | |
| 2 malate → 2 oxaloacetate | 5 | |
| Total | 32 | |


