14: Homework Solutions
- Page ID
- 43948
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Carbohydrate Structure
(01)
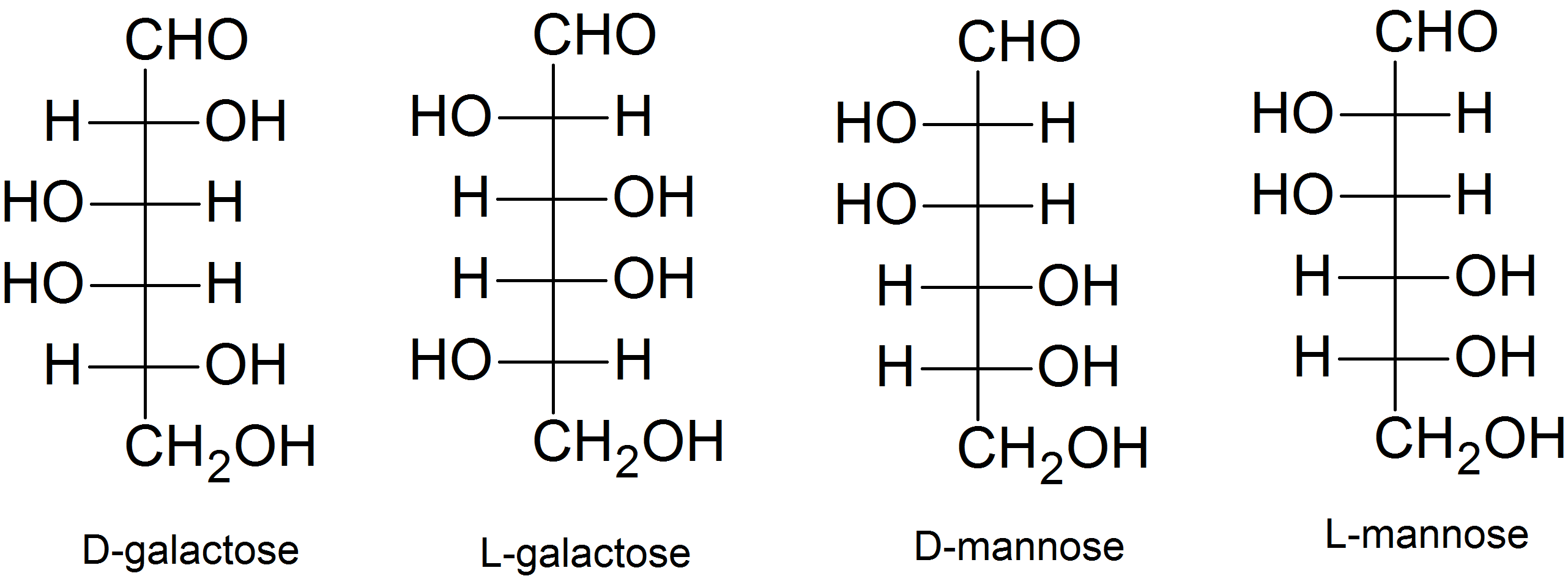
- D-sugar
- D-sugar
- See strucutres above
- No, they differ at C-2 and C-4.
- Diastereomers
(02) The D- or L- classification is determined by the orientation of the hydroxyl group on the chiral carbon furthest from the carbonyl carbon. If this hydroxyl group is positioned on the right, then the sugar is a D-sugar. If this hydroxyl group is positioned on the left, then the sugar is an L-sugar.
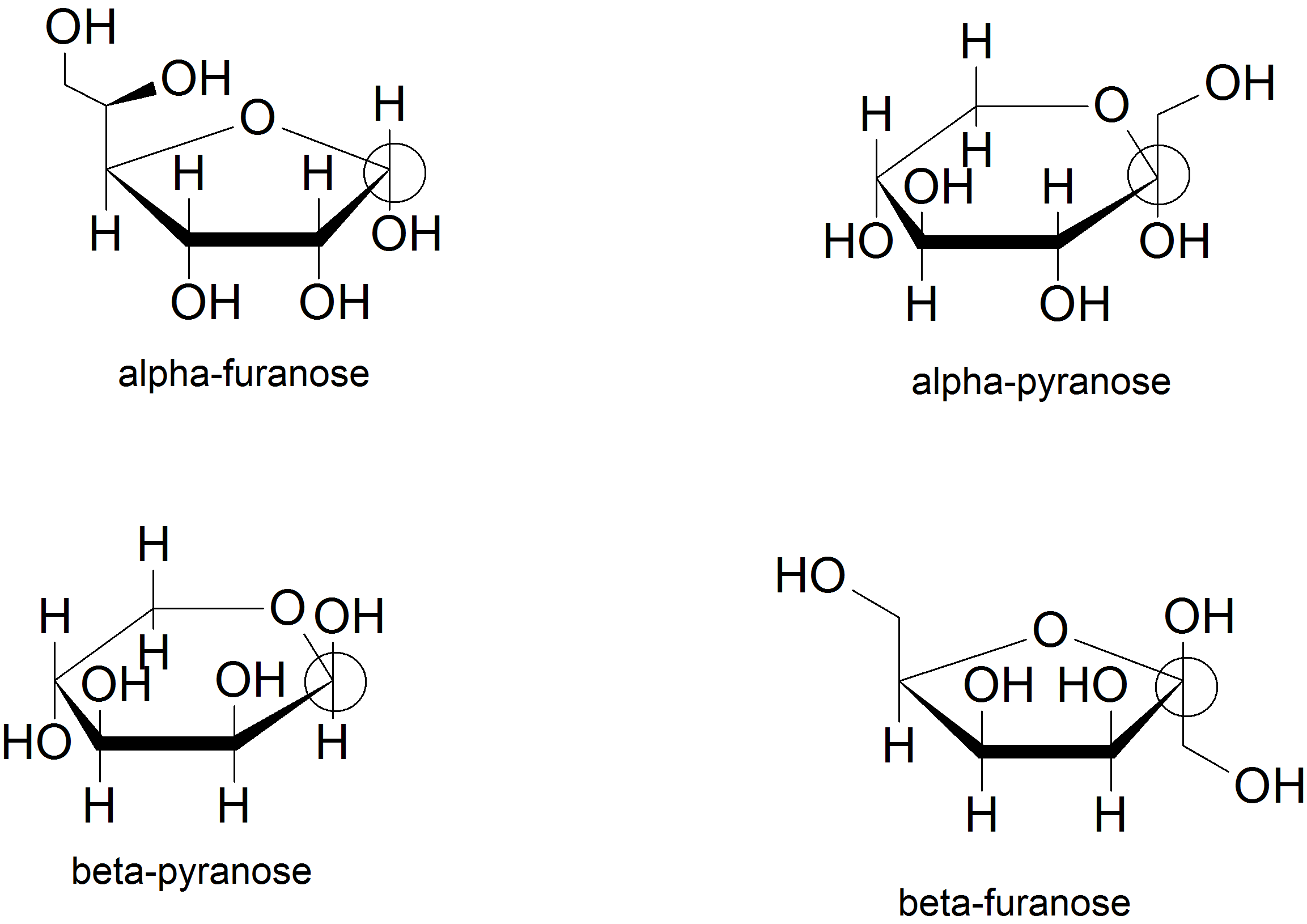
(03) The anomeric carbon is the carbon with two single bonds to two different oxygen atoms. For the alpha anomer, the hydroxyl group on the anomeric carbon points down. For the beta anomer, the hydroxyl group on the anomeric carbon points up.
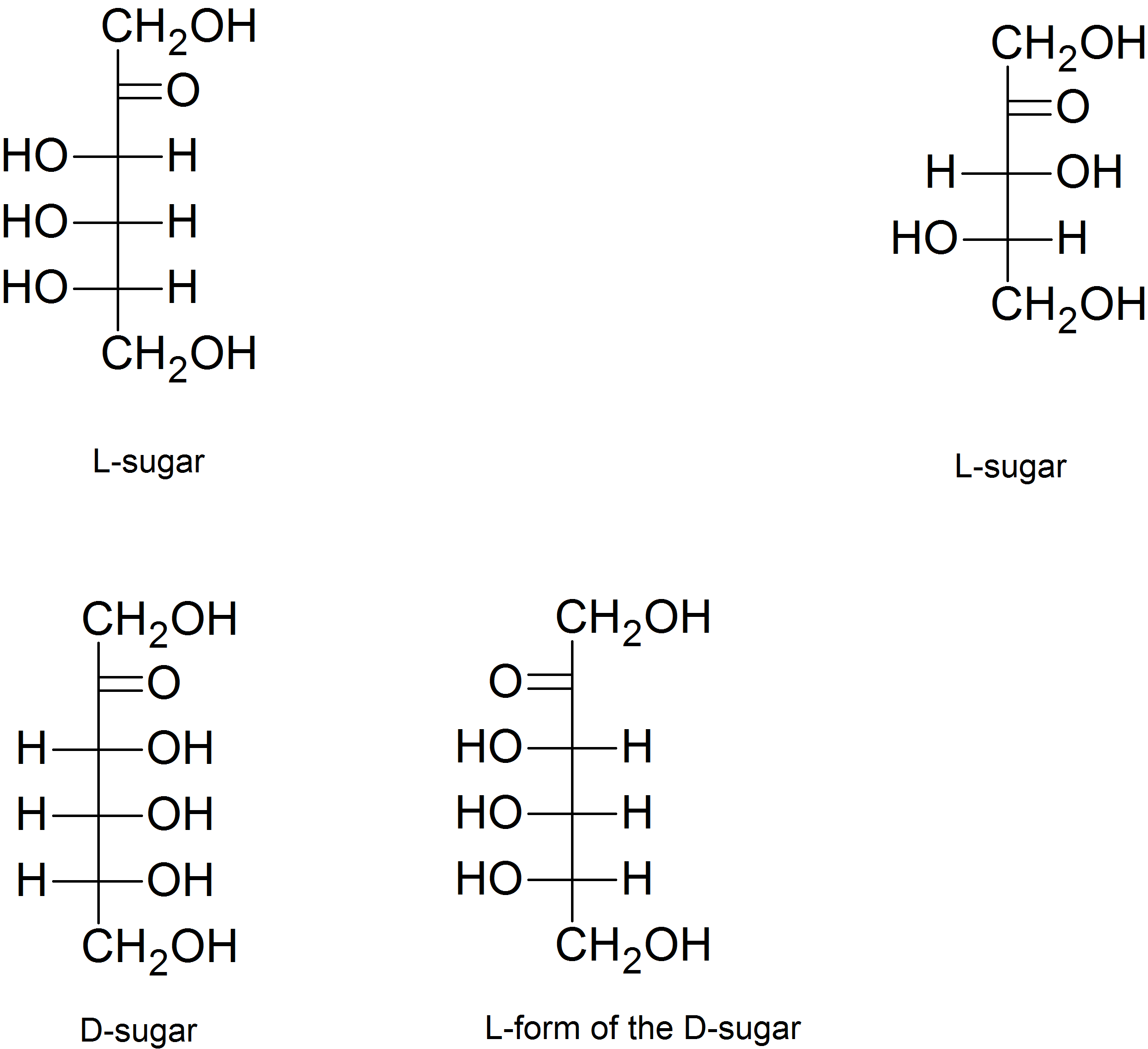
(04)
(05)
- See above
- See above
- See above; enantiomers
- See above; diastereomers
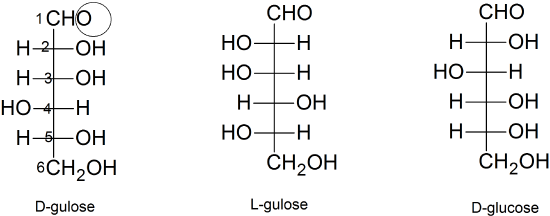
(06)
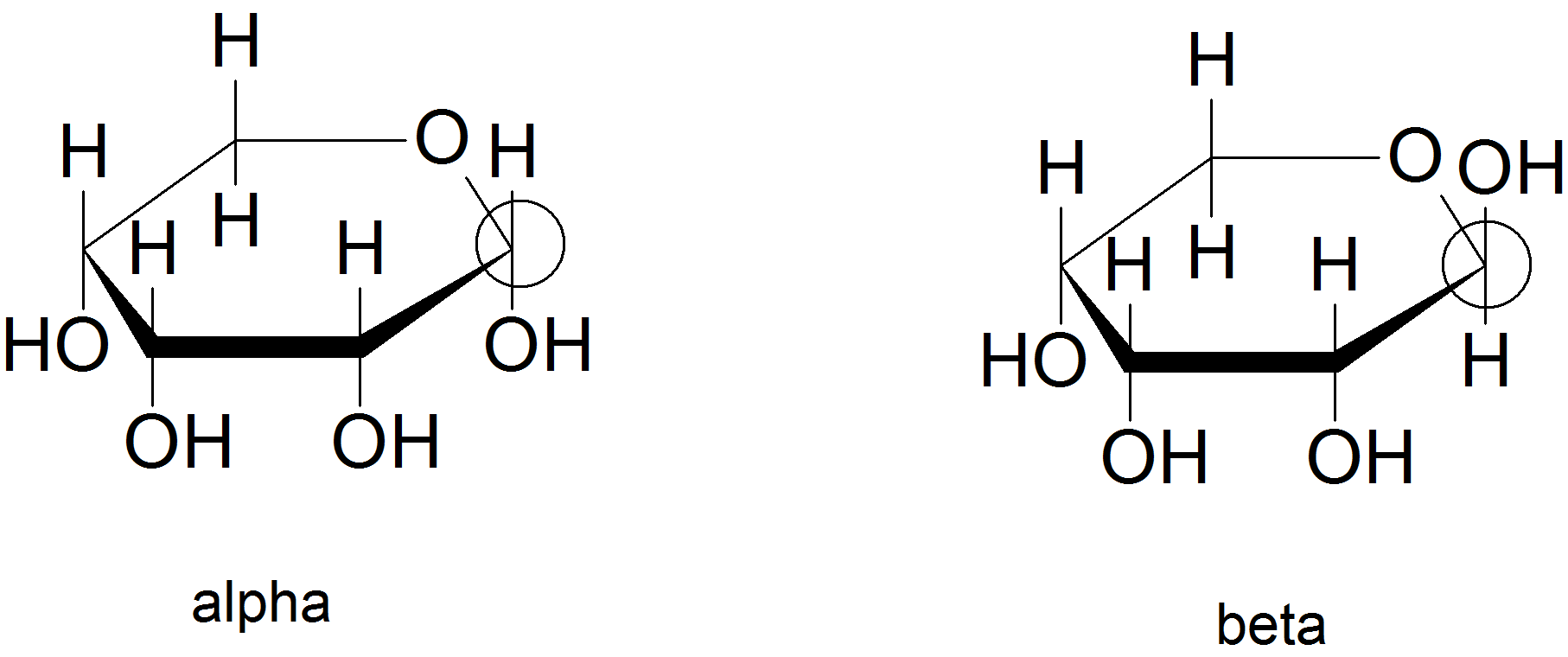
- See above
- See above
- diastereomers - only one chiral carbon is the mirror image
- Yes, because the ring can open for the sugar to oxidize.
- Yes, because the ring of the sugar is constantly opening and closing and the trigonal planar molecular shape of the carbonyl carbon allows both anomers to form.
(07)
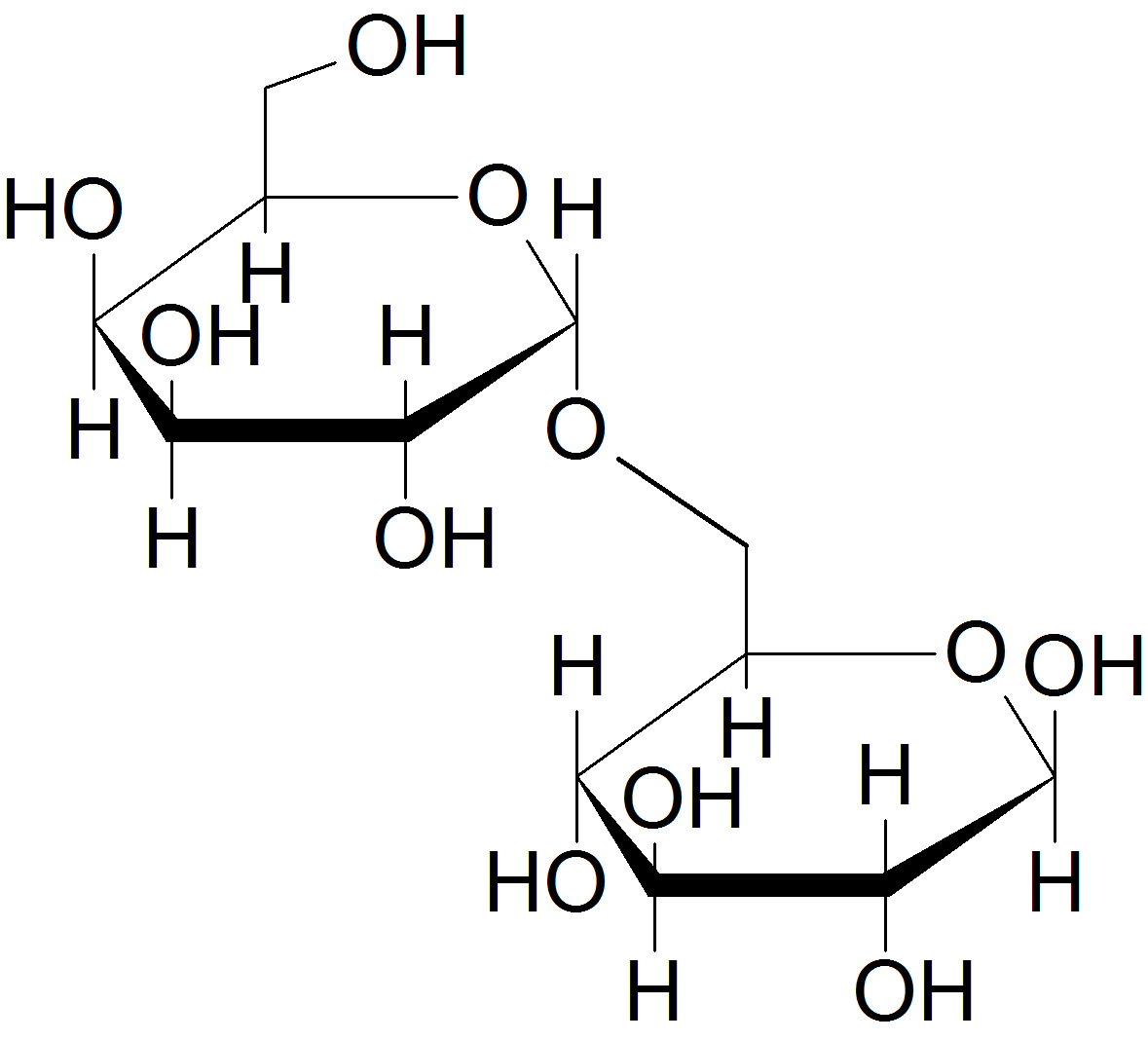
(08)
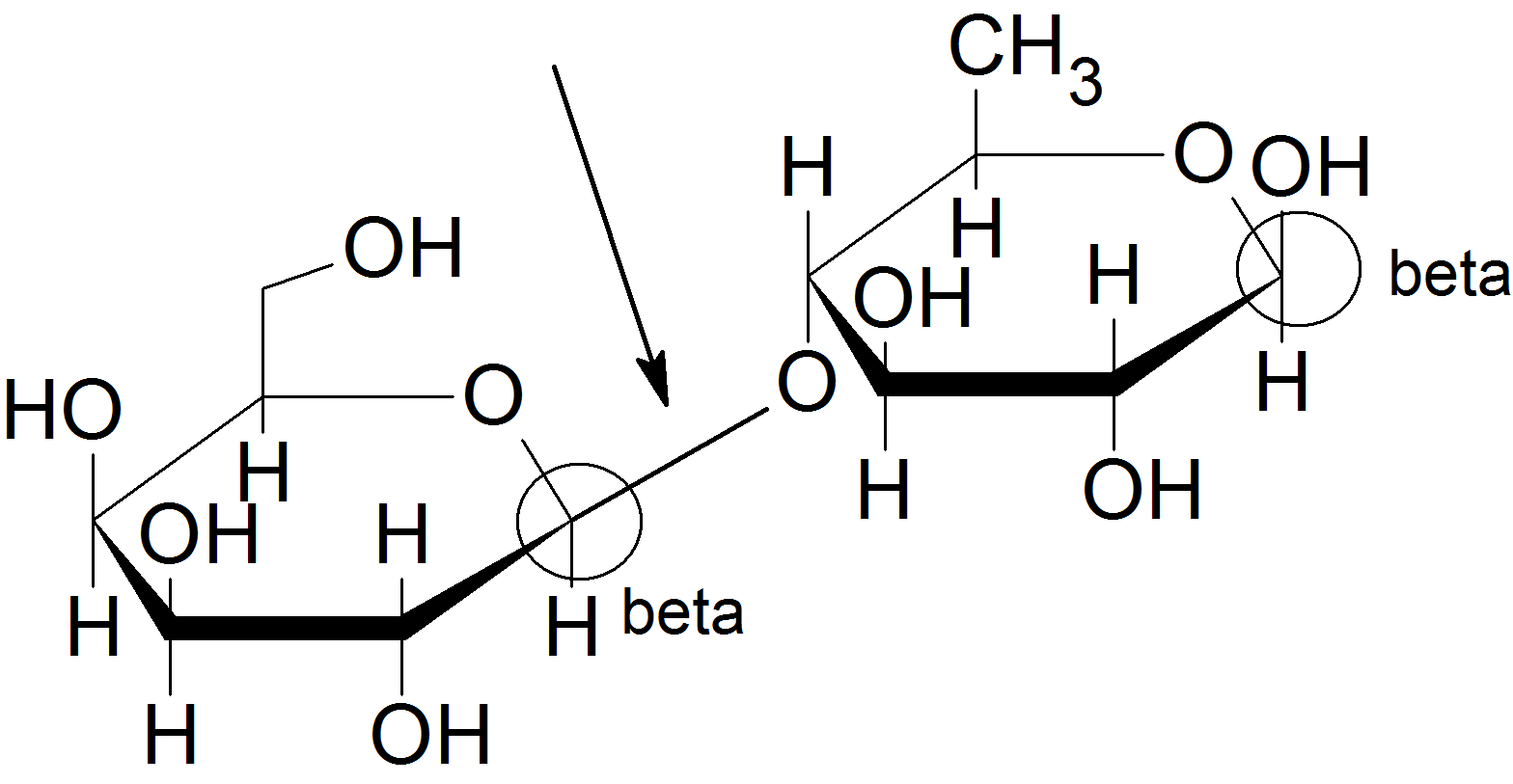
- lactase
- disaccharide.
- see above; yes lactose is a reducing sugar
- See above; No, the glycosidic bond is connected to the anomeric carbon of the sugar on the left (galactose), but it is bonded to a hydroxyl group on C-4 of the sugar on the right (glucose).
- beta-(1,4)
- beta-D-galactose and beta-D-glucose

