13: Homework Solutions
- Page ID
- 43947
Oxidation-Reduction (Redox) Reactions
(01) \[CH_4 + 2 O_2 \rightarrow CO_2 + 2 H_2O\]
- Carbon is oxidized because it lost electrons: \(C^{-4}\) to \(C^{+4}\).
- \(O_2\) is reduced because it gained electrons: \(O^0\) to \(O^{2-}\).
(02)
- "LEO says GER" LEO: Loss of Electrons is Oxidation and Gain of Electrons is Reduction.
- "OIL RIG" Oxidation Is Loss of electrons and Reduction Is Gain of electrons
(03)
- oxidation
- oxidation
- reduction
- reduction
(04) For the following equations, find the oxidation and reduction numbers of each element to determine which compound is oxidized and which is reduced and label them as such. Write the oxidation ½-reaction and the reduction ½-reaction.
a. oxidation half-reaction: \(Al^0 \rightarrow Al^{3+} + 3 e^-\)
reduction half-reaction: \(3 K^+ + 3 e^- \rightarrow 3K^0\) which simplifies to K+ + e- → K0
b. oxidation half-reaction: \(2 H_2^0 \rightarrow 4 H^+ + 4 e^-\) which simplifies to H20 → 2 H+ + 2 e-
reduction half-reaction: \(O_2^0 + 4 e^- \rightarrow 2 O^{2-}\)
c. oxidation half-reaction: \(2 C^{2-} \rightarrow 2 C^{4+} + 12 e^-\) which simplifies to C2- → C4+ + 6 e-
reduction half-reaction: \(3 O_2^0 + 12 e^- \rightarrow 6 O^{2-}\) which simplifies to O20 + 4 e- → 2 O2-
d. oxidation half-reaction: \(3 H_2^0 \rightarrow 6 H^+ + 6 e^-\) which simplifies to H20 → 2 H+ + 2 e-
reduction half-reaction: \(N_2^0 + 6 e^- \rightarrow 2 N^{3-}\)
e. oxidation half-reaction: \(Cu^0 \rightarrow Cu^{2+} + 2 e^-\)
reduction half-reaction: \(2 H^+ + 2 e^- \rightarrow H_2^0 \)
(05) Determine whether each compound below has undergone oxidation or reduction.
- oxidation
- reduction
- reduction
- neither (hydration)
- reduction
(06)
- reducing agent
- oxidizing agent
- oxidizing agent
- reducing agent
(07)
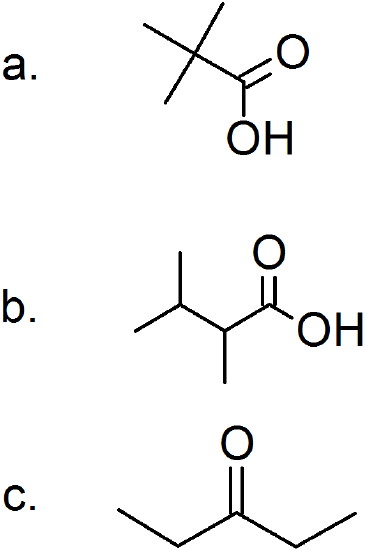
d. no oxidation because there are no hydrogen atoms to lose
e. no oxidation because there are no hydrogen atoms to lose
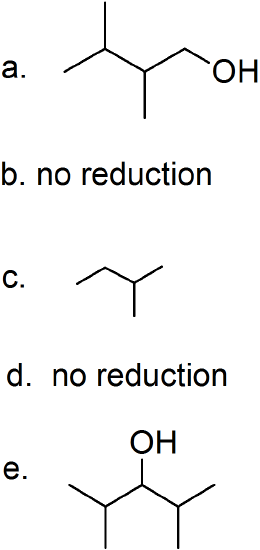
(08) For each of the following organic structures, show what the product would look like after a reduction. If a reaction cannot take place, explain why.
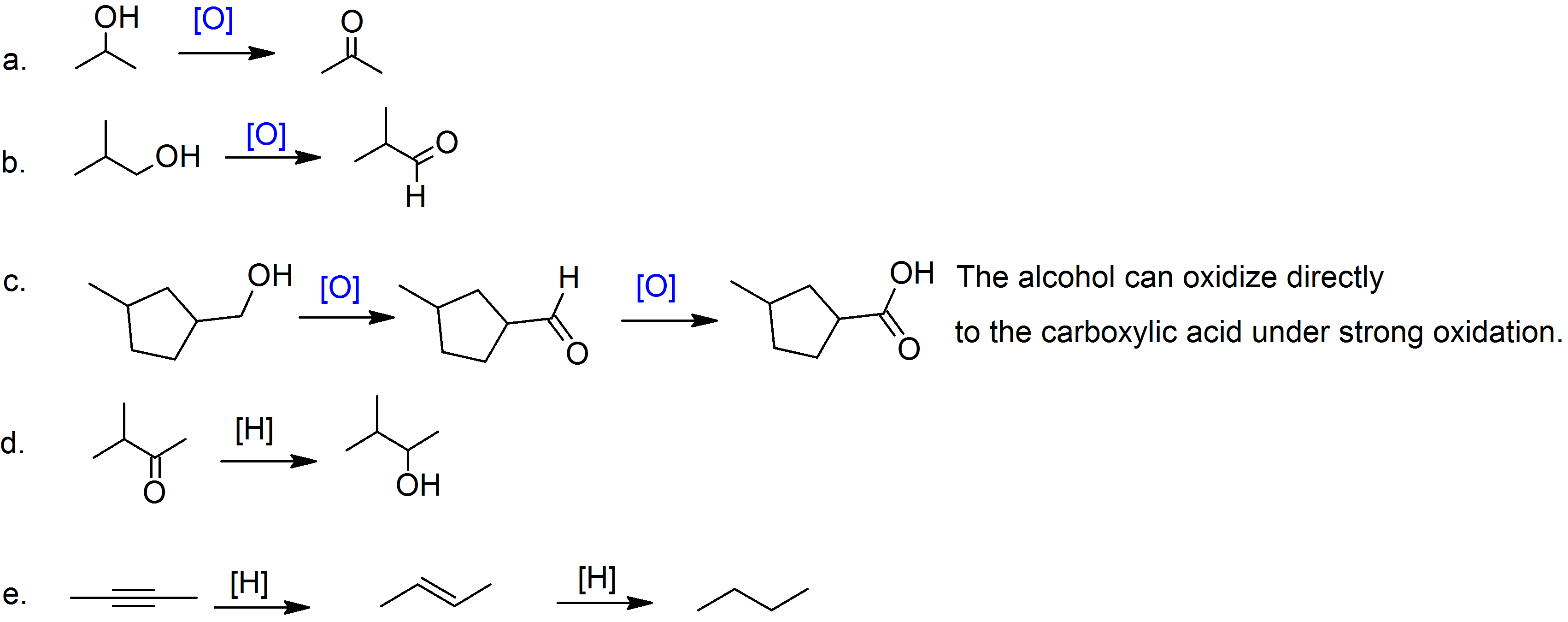
(09)
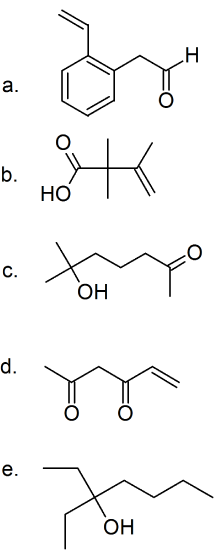
(10)
- T
- F
- T
- T
- T
Hydration-Dehydration Reactions
(11)
- dehydration
- hydration
- dehydration
- dehydration
- dehydration
(12) Complete the following hydration and dehydration reactions and show what products form.
Acyl Group Transfer Reactions
(13)
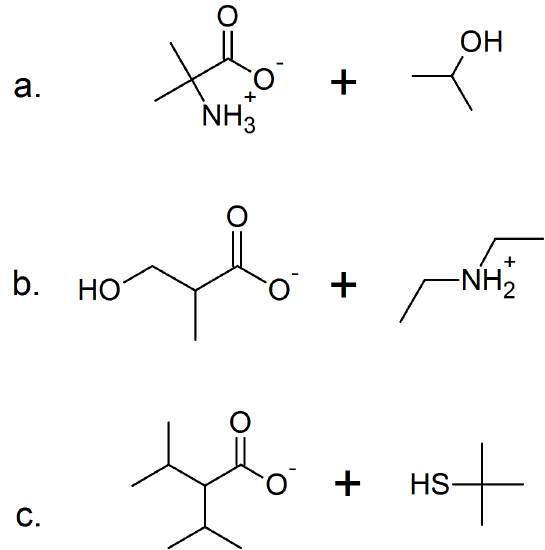
(14)
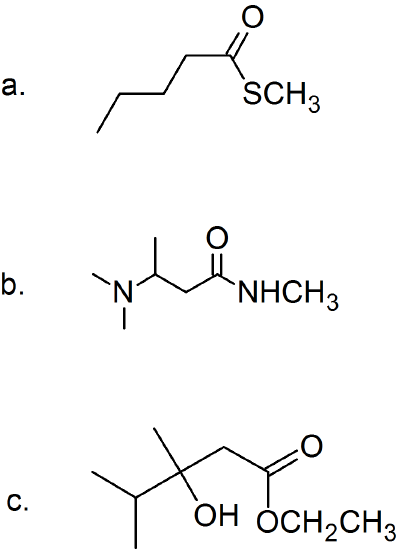
(15) Determine the structure of the products for each esterification reaction.
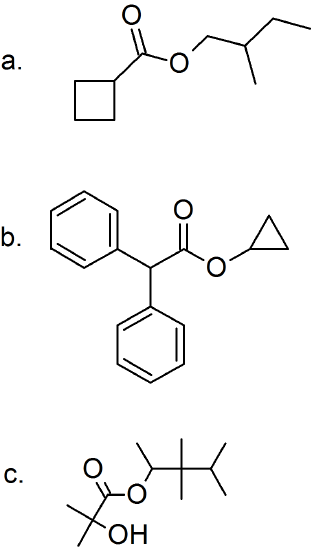
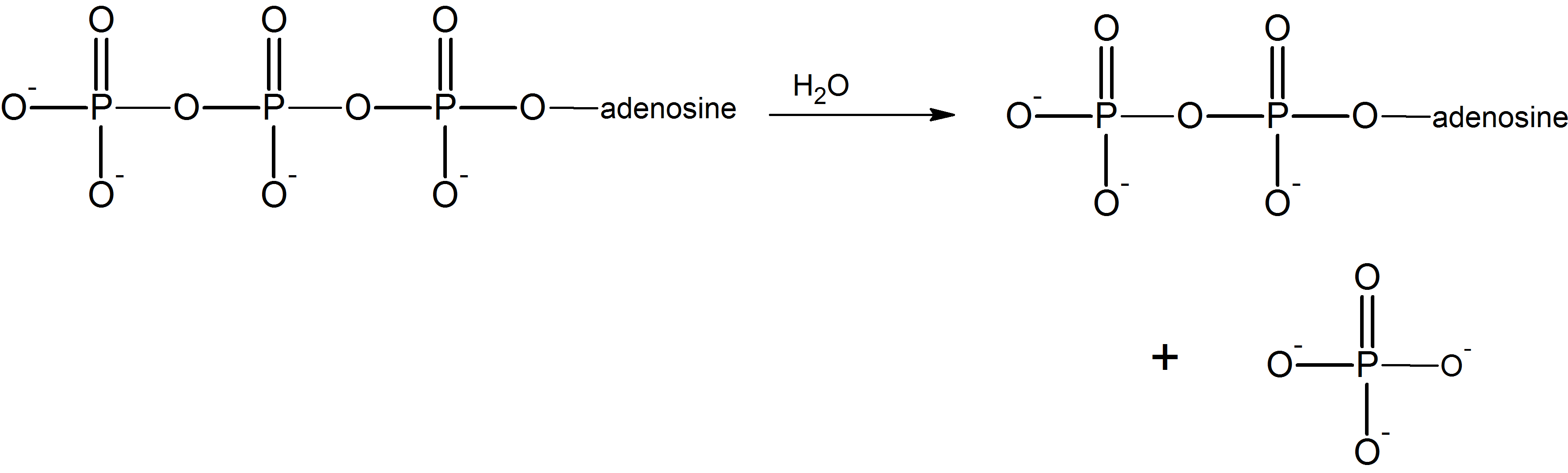
Phosphoryl Group Transfer Reactions
(16)
(17)
- phosphoryl transfer
- hydration
- acyl transfer
- dehydration
- hydrolysis

