12: Acids, Bases, pH and Buffers: Questions
- Page ID
- 43926
Properties of Acids and Bases
(01) According to the Arrhenius definition of acids, an acid gives off what in solution?
According to the Bronsted-Lowry, what is the definition of an acid? What is the definition of a base?
(02) For every given acid or base, write the equation of their reactions with water when they donate or accept one hydrogen ion.
- Hydrocyanic acid
- Carbonate ion
- Ammonium ion
- Sulfuric acid
- Acetic acid
Conjugate Acids and Bases
(03) For each of the following acids, determine the form of their conjugate bases.
- HSO4-
- H2CO3
- HF
- HPO42-
- HCOOH
(04) For each of the following bases, determine the form of their conjugate acids.
- Br -
- NO3-
- S2-
- NH3
- HCO3-
Strengths of Acids and Bases
(05) Nitric acid, HNO3, is a strong acid. Define ‘strong’ acid, and write the equation of nitric acid reacting with water. How does this equation differ from the reaction of a weak acid, such as hydrosulfuric acid (H2S)? Sketch the reaction of hydrosulfuric acid with water to see the difference clearly.
(06) Lithium hydroxide, LiOH, is a strong base. Define ‘strong base’ and write the equation of lithium hydroxide reacting with water. Sketch an example of the reaction of a weak base with water and compare it to the strong base reaction. How do they differ?
(07) Phosphoric acid is one of the few acids that has three acidic hydrogens, and is called ‘triprotic’. Write a chemical equation for phosphoric acid reacting with water and losing one of its hydrogens. Using the products from the first equation, write another equation to show the second hydrogen being lost. Continue in this fashion to show the last hydrogen being lost.
(08) For each chemical reaction, determine whether they are representative of the dissociation reaction of a strong acid, a weak acid, a strong base, or a weak base.
- \(Na(OH)_{(aq)} + H_2O_{(l)} → Na^{+}_{(aq)} + OH^-_{(aq)}\)
- \(HF_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + F^-_{(aq)}\)
- \(H_2PO^-_{4\;(aq)} + H_2O_{(l)} \rightleftharpoons H_3PO_{4\;(aq)} + OH^-_{(aq)}\)
- \(H_2PO^-_{4\;(aq)} + H_2O_{(l)} \rightleftharpoons HPO^{2-}_{4\;(aq)} + H_3O^+_{(aq)}\)
- \(HCl_{(aq)} + H_2O_{(l)} → Cl^-_{(aq)} + H_3O^+_{(aq)}\)
(09) Perform the dissociation reactions for each of the given acids. Use the reaction arrow to tell whether they are strong acids or weak acids. For diprotic acids, show the loss of the first hydrogen ion. Hint: What are the six strong acids?
- HClO4
- HSCN
- HBr
- H2CO3
- CH3COOH
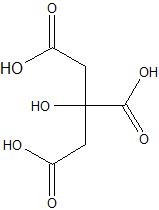
(10) Study the following structure of citric acid. How many acidic groups are contained in this molecule? Circle the acidic hydrogens that can be removed by interactions with bases. Write the three consecutive dissociation reactions using bond-line structures, one reaction for each hydrogen being removed (one at a time).
Acid-Base Equilibria
(11) What is occurring when a reaction is said to be in equilibrium? Write an equation in which water is in equilibrium with a proton and hydroxide. Are the concentrations changing when they are in equilibrium or do they remain constant? Why is using the double-arrows to separate products from reactants so important in equilibrium reactions?
Le Chatelier’s Principle
(12) Observe the following reaction and answer the questions beneath.
\[CH_3COOH_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + CH_3COO^-_{(aq)}\]
- What will the effect on the equilibrium be if the concentration of hydronium ions is increased?
- What will the effect on the equilibrium be if the concentration of acetate is decreased?
- If you increase the concentration of water, will the equilibrium shift left or right?
- If you decrease the concentration of acetic acid, will the equilibrium shift left or right?
(13) A generic example of an equilibrium reaction is shown below. Answer the following questions about what direction equilibrium shift according to Le Chatelier’s principle.
\[A + B \rightleftharpoons C + D + \text{heat}\]
- Removing some of compound \(A\)
- Increasing the temperature
- Adding more of compound \(D\)
- Increasing concentration of compound \(B\)
- Decreasing the temperature
(14) Some processes involve multiple equilibrium equations put together. For example, the carbonation effect that creates soda water involves more than one set of compounds separated by equilibrium arrows, as shown below.
\[CO_{2\;(g)} + H_2O_{(l)} \rightleftharpoons H_2CO_{3\;(aq)} \rightleftharpoons HCO^-_{3\;(aq)} + H^+_{(aq)}\]
a. During hypoventilation, what happens to the hydrogen ion concentration?
b. During hyperventilation, what happens to the hydrogen ion concentration?
Acid-Base Neutralization Reactions
(15) Write out the neutralization reactions for the following pairs. Make sure to balance the equations as necessary.
a. \(Ba(OH)_2\) and \(HNO_3\)
b. \(Ca(OH)_2\) and \(H_2SO_4\)
c. \(RbOH\) and \(HI\)
d. \(KOH\) and \(HClO_4\)
e. \(Mg(OH)_2\) and \(HBr\)
pH
(16) Examine the following pH measures and tell whether they are in the range to be considered acidic, basic or neutral.
- 6.8
- 13.5
- 1.9
- 4.2
- 9.7
(17) Using the formula for converting concentration into pH, determine whether each of the following molarities are acidic, basic or neutral. Remember, -log[H+]
- 1.0 x 10-7 M H+
- 4.7 x 10-12 M H+
- 6.4 x 10-4 M H+
- 7.3 x 10-10 M H+
- 9.8 x 10-2 M H+
(18) Fill in the blank portions of the table.
| [H3O+] | [OH-] | pH | Acidic, Basic or Neutral |
| 1.0 x 10-1 |
|
|
|
|
| 1.0 x 10-4 |
|
|
| 1.0 x 10-7 |
|
|
|
|
| 1.0 x 10-9 |
|
|
| 1.0 x 10-13 |
|
|
|
(19) Given the following sets of information, calculate the requested quantities for each problem.
- [H3O+] = 4.1 x 10-4 M. Find pH, and [OH-]
- [OH-] = 6.6 x 10-9 M. Find pOH, and [H3O+]
- pH = 9.0. Find [H3O+], [OH-] and pOH
- pOH = 12.5. Find [OH-], and [H3O+]
- pOH = 7.0. Find [H3O+], [OH-] and pH
Buffers
(20) What is the definition of a buffer solution? Determine whether or not each pair below can be used to prepare a buffer solution.
- HI and H2SO4
- CH3COOH and CH3COOK
- Ca(OH)2 and H2O
- acetic acid and methylamine
- NH3 and HNO3
(21) Carbonic acid and bicarbonate ion are an conjugate aicd-base pairs. For each of the following compounds, would the carbonic acid or bicarbonate be more likely to react with them and serve as a buffer to prevent pH change?
- HBr
- LiOH
- NH4+
- HCOO-
- CH3NH2
(22) A person who spends a day swimming and diving underwater requires them to hold their breath for long stretches and to do this repeatedly. This is a contrast to the condition of hyperventilation where breaths are taken rapidly. If a person is holding in their inhaled breaths with few breaks in between, what effect does this have on the CO2 levels in their body? Do they increase or decrease? What effect does this increase or decrease have on the pH of the bloodstream?
\[CO_{2\;(g)} + H_2O_{(l)} \rightleftharpoons H_2CO_{3\;(aq)} \rightleftharpoons HCO^-_{3\;(aq)} + H^+_{(aq)}\]

