9.3: Oxidation-Reduction
- Page ID
- 52198
Skills to Develop
- Identify the substance being oxidized and the substance being reduced in an oxidation-reduction reaction.
- Identify the anode and the cathode given a diagram of an electrolysis apparatus that includes the compound being electrolyzed.
- Describe how batteries can produce electrical energy.
Introduction
Electricity is an important form of energy that you use every day. It runs your calculators, cell phones, dishwashers, and watches. This form of energy involves moving electrons through a wire and using the energy of these electrons.
Batteries are one way of producing this type of energy. Many important chemical reactions involve the exchange of one or more electrons, and, therefore we can use this movement of electrons as electricity. These reactions are called oxidation-reduction (or "redox") reactions.
Oxidation and Reduction
Reactions in which electrons are transferred are called oxidation-reduction (or "redox") reactions. There are two parts to these changes: one atom must lose electrons and another atom must gain them. These two parts are described by the terms "oxidation" and "reduction".
Originally, a substance was said to be oxidized when it reacted with oxygen. Today, the word "oxidized" is still used for those situations, but now we have a much broader second meaning for these words. Today, the broader sense of the word oxidation is defined as losing electrons. When a substance loses electrons, its charge will increase. This may feel a bit backwards, but remember that electrons are negative. If an atom loses electrons, it is losing negative particles so its charge will increase.
The other half of this process, the gaining of electrons, also needs a name. When an atom or an ion gains electrons, the charge on the particle goes down. For example, if a sulfur atom whose charge is zero \(\left( 0 \right)\) gains two electrons, its charge becomes \(\left( -2 \right)\) and if an \(\ce{Fe^{3+}}\) ion gains an electron, its charge changes from \(+3\) to \(+2\). In both cases the charge on the particle is reduced by the gain of electrons. Remember that electrons have a negative charge, so gaining electrons will result in the charge decreasing. The word reduction is defined to mean gaining electrons and the reduction of charge.
In chemical systems, these two processes (oxidation and reduction) must occur simultaneously and the number of electrons lost in the oxidation must be the same as the number of electrons gained in the reduction. In oxidation-reduction reactions, electrons are transferred from one substance to another. Here's an example of an oxidation-reduction reaction.
\[2 \ce{Ag^+} \left( aq \right) + \ce{Cu} \left( s \right) \rightarrow 2 \ce{Ag} \left( s \right) + \ce{Cu^{2+}} \left( aq \right)\]
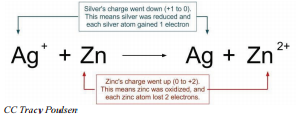 In this reaction, the silver ions are gaining electrons to become silver atoms. Therefore, the silver ions are being reduced and the charge of silver is decreasing. The copper atoms are losing electrons to become copper \(+2\) ions and are therefore, being oxidized and the charge of copper is increasing. Whenever a chemical reaction involves electrons being transferred from one substance to another, the reaction is an oxidation-reduction reaction (or a redox reaction).
In this reaction, the silver ions are gaining electrons to become silver atoms. Therefore, the silver ions are being reduced and the charge of silver is decreasing. The copper atoms are losing electrons to become copper \(+2\) ions and are therefore, being oxidized and the charge of copper is increasing. Whenever a chemical reaction involves electrons being transferred from one substance to another, the reaction is an oxidation-reduction reaction (or a redox reaction).
Half-equations are very helpful in discussing and analyzing processes but half-reactions cannot occur as they appear. The half-reactions for the reaction above would be:
\[2 \ce{Ag^+} \left( aq \right) + 2 \ce{e^-} \rightarrow 2 \ce{Ag} \left( s \right)\]
\[\ce{Cu} \left( s \right) \rightarrow \ce{Cu^{2+}} \left( aq \right) + 2 \ce{e^-}\]
Both oxidation and reduction must occur at the same time so the electrons are donated and absorbed nearly simultaneously. The two half-reactions may be added together to represent a complete reaction. In order to add the half-reactions, the number of electrons donated and the number of electrons accepted must be equal.
Example 9.3.1
For each reaction, identify what is oxidized and what is reduced.
a) \(\ce{Zn} + \ce{HCl} \rightarrow \ce{H_2} + \ce{ZnCl_2}\)
b) \(\ce{Fe} + \ce{O_2} \rightarrow \ce{Fe_2O_3}\)
c) \(\ce{NaBr} + \ce{I_2} \rightarrow \ce{NaI} + \ce{Br_2}\)
Solution:
In order to determine what is being oxidized and reduced, we must look at charges of atoms and see if they increase or decrease. (Remember, elements have no charge. In a compound, we can use our periodic table and what we learned in Chapter 4 to assign charges.) If the charge increases, the atom was oxidized. If the charge decreases, the atom was reduced.
a) This reaction written with charges is:
\[\ce{Zn^0} + \ce{H^+Cl^-} \rightarrow \ce{H_2^0} + \ce{Zn^{2+}Cl_2^-}\]
\(\ce{Zn}\) is oxidized because it went from \(0\) to \(+2\). \(\ce{H}\) is reduced because it went from \(+1\) to \(0\). \(\ce{Cl}\) was neither oxidized nor reduced.
b) This reaction written with charges is:
\[\ce{Fe^0} + \ce{O_2^0} \rightarrow \ce{Fe_2^{3+}O_3^{2-}}\]
\(\ce{Fe}\) is oxidized because it went from \(0\) to \(+3\). \(\ce{O}\) is reduced because it went from \(0\) to \(-2\).
c) This reaction written with charges is:
\[\ce{Na^+Br^-} + \ce{I_2^0} \rightarrow \ce{Na^+I^-} + \ce{Br_2^0}\]
\(\ce{Br}\) is oxidized because it went from \(-2\) to \(0\). \(\ce{I}\) is reduced because it went from \(0\) to \(-1\). \(\ce{Na}\) was neither oxidized nor reduced as it stayed \(+1\) the whole time.
Batteries
Batteries are devices that use chemical reactions to produce electrical energy. These reactions occur because the produces contain less potential energy in their bonds than the reactants. The energy produced from excess potential energy not only allows the reaction to occur but also often gives off energy to the surroundings. Some of these reactions can be physically arranged so the energy given off is given off in the form of an electric current. These are the type of reactions that occur inside batteries. When a reaction is arranged to produce an electric current as it runs, the arrangement is called an electrochemical cell or a Galvanic Cell.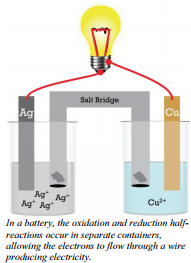
If a strip of copper is placed in a solution of silver nitrate, the following reaction takes place:
\[2 \ce{Ag^+} \left( aq \right) + \ce{Cu} \left( s \right) \rightarrow 2 \ce{Ag} \left( s\right) + \ce{Cu^{2+}} \left( aq \right)\]
In this reaction, copper atoms are donating electrons to silver ions so the silver ions are reduced to silver atoms and copper atoms are oxidized to copper (II) ions.
As the reaction occurs, an observer would see the solution slowly turn blue (\(\ce{Cu^{2+}}\) ions are blue in solution) and a mass of solid silver atoms would build up on the copper strip.
The reaction we just described is not set up in such a way as to produce electricity. It is true that electrons are being transferred, but to produce electricity we need electrons flowing through a wire so we can use the energy of these electrons. This reactions, \(2 \ce{Ag^+} \left( aq \right) + \ce{Cu} \left( s \right) \rightarrow 2 \ce{Ag} \left( s \right) + \ce{Cu^{2+}} \left( aq \right)\), is one that could be arranged to produce electricity. To do this, the two half-reactions (oxidation and reduction) must occur in separate compartments and the separate compartments must remain in contact through an ionic solution and an external wire.
In this electrochemical cell, the copper metal must be separated from the silver ions to avoid a direct reaction. Each electrode in its solution could be represented by a half-reaction.
\[\ce{Cu} \rightarrow \ce{Cu^{2+}} + 2 \ce{e^-}\]
\[2 \ce{Ag^+} + 2 \ce{e^-} \rightarrow 2 \ce{Ag}\]
The wire connects the two halves of the reaction, allowing electrons to flow from one metal strip to the other. In this particular example, electrons will flow from the copper electrode (which is losing electrons) into the silver electrode (which is where the silver ions gain the electrons). The cell produce electricity through the wire and will continue to do so as long as there are sufficient reactants (\(\ce{Ag^+}\) and \(\ce{Cu}\)) to continue the reaction.
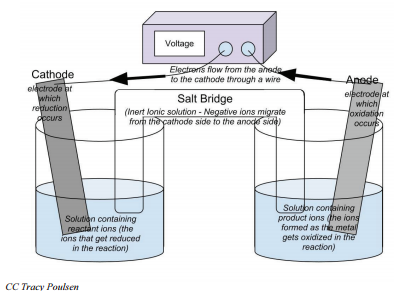
Electrochemical cells will always have two electrodes, the pieces of metal where electrons are gained or lost. (In this example, the strip of \(\ce{Ag}\) metal and \(\ce{Cu}\) metal are the electrodes.) The electrode where reduction occurs and electrons are gained is called the cathode. The electrode where oxidation occurs and electrons are lost is called the anode. Electrons will always move from the anode to the cathode. The electrons that pass through the external circuit can do useful work such as lighting lights, running cell phones, and so forth.
If the light bulb is removed from the circuit with the electrochemical cell and replaced with a voltmeter, the voltmeter will measure the voltage (electrical potential energy per unit charge) of the combination of half-cells. The size of the voltage produced by a cell depends on the temperature, the metals used for electrodes, and the concentrations of the ions in the solutions. If you increase the concentration of the reactant ion (not the produce ion), the reaction rate will increase and so will the voltage.
It may seem complicated to construct an electrochemical cell because of all their complexities. Electrochemical cells are actually easy to make and sometimes even occur accidentally. If you take two coins of different denomination and push them part way through the peel of a whole lemon and then connect the two coins with a wire, a small electric current will flow.
Electrolysis
So far we have discussed how electricity can be produced from chemical reactions in batteries. Some reactions will, instead, use electricity to get a reaction to occur. In these reactions, electrical energy is given to the reactants causing them to react to form the products. These reactions have many uses.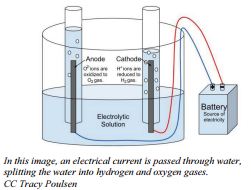
Electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis reactions will not run unless energy is put into the system from outside. In the case of electrolysis reactions, the energy is provided by the battery.
If electrodes connected to battery terminals are placed in liquid sodium chloride, the sodium ions will migrate toward the negative electrode and be reduced while the chloride ions migrate toward the positive electrode and are oxidized. The processes that occur at the electrodes can be represented by what are called half-equations.
Reduction occurs at the positive electrode:
\[\ce{Na^+} + \ce{e^-} \rightarrow \ce{Na}\]
Oxidation occurs at the negative electrode:
\[2 \ce{Cl^-} \rightarrow \ce{Cl_2} + 2 \ce{e^-}\]
The overall reaction for this reaction is:
\[2 \ce{Na^+} + 2 \ce{Cl^-} \rightarrow 2 \ce{Na} + \ce{Cl_2}\]
With appropriate treatment from the battery, it is possible to get the metal being reduced in an electrolysis process to adhere strongly to the electrode. The use of electrolysis to coat one material with a layer of metal is called electroplating. Usually, electroplating is used to cover a cheap metal with a layer of more expensive and more attractive metal. Many girls buy jewelry that is plated in gold. Sometimes, electroplating is used to get a surface metal that is a better conductor of electricity. When you wish to have the surface properties of gold (attractive, corrosion resistant, or good conductor) but you don't want to have the great cost of making the entire object out of solid gold, the answer may be to use cheap metal to make the object and then electroplate a thin layer of gold on the surface.
To silver plate an object like a spoon (silverware that's plated is less expensive than pure silver), the spoon is placed in the position of the cathode in an electrolysis set up with a solution of silver 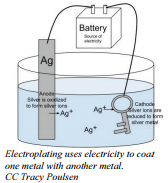 nitrate. When the current is turned on, the silver ions will migrate through the solution, touch the cathode (spoon) and adhere to it. With enough time and care, a layer of silver can be plated over the entire spoon. The anode for this operation would often be a large piece of silver from which silver ions would be oxidized and these ions would enter the solution. This is a way of ensuring a steady supply of silver ions for the plating process.
nitrate. When the current is turned on, the silver ions will migrate through the solution, touch the cathode (spoon) and adhere to it. With enough time and care, a layer of silver can be plated over the entire spoon. The anode for this operation would often be a large piece of silver from which silver ions would be oxidized and these ions would enter the solution. This is a way of ensuring a steady supply of silver ions for the plating process.
Half-reaction at the cathode: \(\ce{Ag^+} + \ce{e^-} \rightarrow \ce{Ag}\)
Half-reaction at the anode: \(\ce{Ag} \rightarrow \ce{Ag^+} + \ce{e^-}\)
Some percentage of the gold and silver jewelry sold is electroplated. The connection points in electric switches are often gold plated to improve electrical conductivity and most of the chromium pieces on automobiles are chromium plated.
Lesson Summary
- Reactions in which there is a transfer of electrons is said to be an oxidation-reduction reaction or a redox reaction.
- A substance that loses electrons is said to be oxidized, and the substance that gains electrons is said to be reduced.
- Redox reactions can be used in electrochemical cells to produce electricity.
- Electrochemical cells are composed of an anode and cathode in two separate solutions. These solutions are connected by a salt bridge and a conductive wire.
- An electric current consists of a flow of charged particles.
- The electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode.
- In electroplating, the object to be plated is made the cathode.
Vocabulary
- Oxidation: A loss of electrons, resulting in an increased charge or oxidation number.
- Reduction: Gaining electrons, resulting in a decreased charge or oxidation number.
- Battery: A group of two or more cells that produces an electric current.
- Anode: The electrode at which oxidation occurs.
- Cathode: The electrode at which reduction occurs.
- Electrochemical cell: An arrangement of electrodes and ionic solutions in which a redox reaction is used to make electricity (a.k.a., a battery)
- Electrolysis: A chemical reaction brought about by an electric current.
- Electroplating: A process in which electrolysis is used as a means of coating an object with a layer of metal.
Further Reading/Supplemental Links
- Wikipedia: How Batteries Work http://en.wikipedia.org/wiki/Battery...batteries_work
- Battery simulation: Galvanic Cells http://www.mhhe.com/physsci/chemistr...sh/galvan5.swf
- http://learner.org/resources/series61.html The learner.org website allows users to view streaming videos of the Annenberg series of chemistry videos. You are required to register before you can watch the videos but there is no charge. The website has one video that relates to this lesson called The Busy Electron.
- The principles of electrochemical cell design are explained through batteries, sensors, and a solar-powered car. The Busy Electron (http://www.learner.org/vod/vod_window.html?-id=807)
- Dictionary of Scientific and Technical Terms, Sybil P. Parker, Editor in Chief, McGraw-Hill, 1994.
- http://academic.pgcc.edu/~ssinex/E_cells.pdf
- http://en.wikipedia.org
- Therese Forsythe. Illustration of an Endothermic Reaction. CC-BY-SA.
- Richard Parsons. Sodium chloride crystal, NaCl. CC-BY-SA.

