7.9: The Effects of Applying Stress to Reactions at Equilibrium
- Page ID
- 51451
Skills to Develop
- State Le Châtelier's Principle.
- Describe the effect of concentration on an equilibrium system.
- Describe the effect of temperature as a stress on an equilibrium system.
When a reaction has reached equilibrium with a given set of conditions, if the conditions are not changed, the reaction will remain at equilibrium forever. The forward and reverse reactions continue at the same equal and opposite rates and the macroscopic properties remain constant.
It is possible, however, to disturb that equilibrium by changing conditions. For example, you could increase the concentration of one of the products, or decrease the concentration of one of the reactants, or change the temperature. When a change of this type is made in a reaction at equilibrium, the reaction is no longer in equilibrium. When you alter something in a reaction at equilibrium, chemists say that you put stress on the equilibrium. When this occurs, the reaction will no longer be in equilibrium and the reaction itself will begin changing the concentrations of reactants and products until the reaction comes to a new position of equilibrium. How a reaction will change when a stress is applied can be explained and predicted. That's the topic of this section.
Le Châtelier's Principle
In the late 1800's, a chemist by the name of Henry-Louis Le Châtelier was studying stresses that were applied to chemical equilibria. He formulated a principle from this research and, of course, the principle is called Le Châtelier's Principle. Le Châtelier's Principle states that when a stress is applied to a system at equilibrium, the equilibrium will shift in a direction to partially counteract the stress and once again reach equilibrium.
that when a stress is applied to a system at equilibrium, the equilibrium will shift in a direction to partially counteract the stress and once again reach equilibrium.
Le Châtelier's principle is not an explanation of what happens on the molecular level to cause the equilibrium shift, it is simply a quick way to determine which way the reaction will run in response to a stress applied to the system at equilibrium.
Effect of Concentration Changes on a System at Equilibrium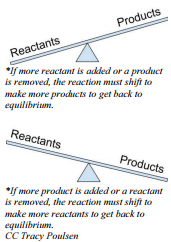
For instance, if a stress is applied by increasing the concentration of a reactant, the reaction will adjust in such a way that the reactants and products can get back to equilibrium. In this case, you made it so there is too much reactant. The reaction will use up some of the reactant to make more product. We would say the reaction "shifts to the products" or "shifts to the right". If you increase the concentration of a product, you have the opposite effect. The reaction will use up some of the product to make more reactant. The reaction "shifts to the reactants" or "shifts to the left".
What if we remove some reactant or product? If a stress is applied by lowering a reactant concentration, the reaction will try to replace some of the missing reactant. It uses up some of the product to make more reactant, and the reaction "shifts to the reactants". If a stress is applied by reducing the concentration of a product, the equilibrium position will shift toward the products.
Example 7.9.1
For the reaction: \(\ce{SiCl_4} \left( g \right) + \ce{O_2} \left( g \right) \rightleftharpoons \ce{SiO_2} \left( s \right) + 2 \ce{Cl_2} \left( g \right)\), what would be the effect on the equilibrium system if:
a) \(\left[ \ce{SiCl_4} \right]\) increases
b) \(\left[ \ce{O_2} \right]\) increases
c) \(\left[ \ce{Cl_2} \right]\) increases
Solution:
a) \(\left[ \ce{SiCl_4} \right]\) increases: The equilibrium would shift to the right
b) \(\left[ \ce{O_2} \right]\) increases: The equilibrium would shift to the right
c) \(\left[ \ce{Cl_2} \right]\) increases: The equilibrium would shift left
Example 7.9.2
Here's a reaction at equilibrium. \(\text{A} \left( aq \right) + \text{B} \left( aq \right) \rightleftharpoons \text{C} \left( aq \right) + \text{D} \left( aq \right)\)
a) Which way will the equilibrium shift if you add some \(\text{A}\) to the system without changing anything else?
b) Which way will the equilibrium shift if you add some \(\text{C}\) to the system without changing anything else?
Solution:
a) The equilibrium will shift toward the products (forward).
b) The equilibrium will shift toward the reactants (backward).
The Effect of Changing Temperature on a System at Equilibrium
Le Châtelier's principle also correctly predicts the equilibrium shift when systems at equilibrium are heated and cooled. An increase in temperature is the same as adding heat to the system. Consider the following equilibrium:
\[2 \ce{SO_2} \left( g \right) + \ce{O_2} \left( g \right) \rightleftharpoons 2 \ce{SO_3} \left( g \right) \: \: \: \: \: \Delta H = -191 \: \text{kJ}\]
We will learn more about this later, but \(\Delta H\) has to do with the change in energy, usually heat, for this reaction. The negative sign \(\left( - \right)\) in the \(\Delta H\) indicates that energy is being given off. This equation can also be written as:
\[2 \ce{SO_2} \left( g \right) + \ce{O_2} \left( g \right) \rightleftharpoons 2 \ce{SO_3} \left( g \right) + 191 \: \text{kJ of heat}\]
What's important to remember about increasing the temperature of an equilibrium system, is the energy can be thought as just another product or reactant. In this example, you can clearly see that the \(191 \: \text{kJ}\) are a product. Therefore when the temperature of this system is raised, heat is being added and the effect will be the same as increasing any other product. Increasing a product causes the reaction to use up some of the products to make more reactants. And, if the temperature for this equilibrium system is lowered, the equilibrium will shift to make up for this stress. When the temperature is decreased for this reaction, the reaction will shift toward the products in an attempt to counteract the decreased temperature.Therefore,the \(\left[ \ce{SO_3} \right]\) will increase and the \(\left[ \ce{SO_2} \right]\) and \(\left[ \ce{O_2} \right]\) will decrease.
In some reactions, though, heat is a reactant. These reactions are called endothermic reactions. These reactions would have the opposite effect. If heat is a reactant, adding heat adds a reactant and the reaction will shift towards the products. If heat is removed (by lowering the temperature) from an endothermic reaction, a reactant is removed and the reaction will shift to make more reactants.

Example 7.9.3
Predict the effect on the equilibrium position if the temperature is increased in each of the following.
a) \(\ce{H_2} \left( g \right) + \ce{CO_2} \left( g \right) \rightleftharpoons \ce{CO} \left( g \right) + \ce{H_2O} \left( g \right) \: \: \: \: \: \Delta H = +40 \: \text{kJ/mol}\)
b) \(2 \ce{SO_2} \left( g \right) + \ce{O_2} \left( g \right) \rightleftharpoons 2 \ce{SO_3} \left( g \right) + \text{energy}\)
Solution:
a) The reaction is endothermic, because \(\Delta H\) is positive, meaning heat is a reactant. We would write:
\[40 \: \text{kJ} + \ce{H_2} \left( g \right) + \ce{CO_2} \left( g \right) \rightleftharpoons \ce{CO} \left( g \right) + \ce{H_2O} \left( g \right)\]
With an increase in temperature for an endothermic reaction, the reactions will shift right producing more products.
b) The reaction is exothermic, meaning the heat is a product. With an increase in temperature for an exothermic reaction, the reactions will shift left producing more reactants.
The Haber Process
Let's look at a particularly useful reaction and how chemists applied Le Châtelier's Principle to make more of a desired product. The reaction between nitrogen gas and hydrogen gas can produce ammonia, \(\ce{NH_3}\). Under normal conditions, this reaction does not produce very much ammonia. Early in the 20\(^\text{th}\) century, the commercial use of this reaction was too expensive because of the small yield of ammonia. The reaction is as follows:
\[\ce{N_2} \left( g \right) + 3 \ce{H_2} \left( g \right) \rightleftharpoons 2 \ce{NH_3} \left( g \right) + \text{energy}\]
Fritz Haber, a German chemist working in the early years of the 20\(^\text{th}\) century, applied Le Châtelier's principle to help solve this problem. Decreasing the concentration of ammonia, for instance, by immediately removing it from the reaction container will cause the equilibrium to shift to the right and continue to produce more product.
One more factor that will affect this equilibrium system is the temperature. Since the forward reaction is exothermic (heat is released as a product), lowering the temperature will once again shift the equilibrium system to the right and increase the ammonia that is produced. Specifically the conditions that were found to produce the greatest yield of ammonia are \(550^\text{o} \text{C}\) (in commercial situations this is a "low" temperature) and \(250 \: \text{atm}\) of pressure. Once the equilibrium system is producing the ammonia, the product is removed, cooled, and dissolved in water.
Lesson Summary
- Increasing the concentration of a reactant causes the equilibrium to shift to the right, producing more products.
- Increasing the concentration of a product causes the equilibrium to shift to the left, producing more reactants.
- Decreasing the concentration of a reactant causes the equilibrium to shift to the left, producing more reactants.
- Decreasing the concentration of a product causes the equilibrium to shift to the right, producing more products.
- For a forward exothermic reaction, an increase in temperature shifts the equilibrium toward the reactant side whereas a decrease in temperature shifts the equilibrium toward the product side.
Vocabulary
- Le Châtelier's Principle: Applying a stress to an equilibrium system causes the equilibrium position to shift to offset that stress and regain equilibrium
- Exothermic reaction: A reaction in which heat is released, or is a product of a reaction.
- Endothermic reaction: A reaction in which heat is absorbed, or is a reactant of a reaction.
- Catalyst: A substance that increases the rate of a chemical reaction but is, itself, left unchanged at the end of the reaction.
Further Reading/Supplemental Links
- http://en.wikipedia.org/wiki
- Tutorial: Le Châtlier's Principle: http://www.mhhe.com/physsci/chemistr...sh/lechv17.swf

