7.4: Balancing Chemical Equations
- Page ID
- 50635
Skills to Develop
- Explain the roles of subscripts and coefficients in chemical equations.
- Balance a chemical equation when given the unbalance equation.
- Explain the role of the Law of Conservation of Mass in a chemical reaction.
Even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not disappear nor do new atoms appear to form the products. In chemical reactions, atoms are never created or destroyed. The same atoms that were present in the reactants are present in the products - they are merely reorganized into different arrangements. In a complete chemical equation, the two sides of the equation must be present on the reactant and the product sides of the equation.
Coefficients and Subscripts
There are two types of numbers that appear in chemical equations. There are subscripts, which are part of the chemical formulas of the reactants and products and there are coefficients that are placed in front of the formulas to indicate how many molecules of that substance is used or produced.
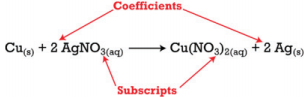
The subscripts are part of the formulas and once the formulas for the reactants and products are determined, the subscripts may not be changed. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of aqueous copper (II) nitrate and two atoms of solid silver.
Once you have written a symbolic equation from words, it is important to balance the equation. It is very important to note that these steps must be carried out in the correct order. You must have to correct formulas for your reactants and products before you can use coefficients to balance the equation.
When you learned how to write formulas, it was made clear that when only one atom of an element is present, the subscript of "1" is not written - so that when no subscript appears for an atom in a formula, you read that as one atom. The same is true in writing balanced chemical equations. If only one atom or molecule is present, the coefficient of "1" is omitted. Coefficients are inserted into the chemical equation in order to balance it; that is, to make the total number of each atom on the two sides of the equation equal. Equation balancing is accomplished by changing coefficients, never by changing subscripts.
Example \(\PageIndex{1}\)
Balance the following skeletal equation. (The term "skeletal equation" refers to an equation that has the correct formulas but has not yet had the proper coefficients added.)
\[\ce{Fe(NO_3)_3} + \ce{NaOH} \rightarrow \ce{Fe(OH)_3} + \ce{NaNO_3} \nonumber\]
Solution: We can balance the hydroxide ion by inserting a coefficient of 3 in front of the \(\ce{NaOH}\) on the reactant side.
\[\ce{Fe(NO_3)_3} + 3 \ce{NaOH} \rightarrow \ce{Fe(OH)_3} + \ce{NaNO_3} \nonumber\]
Then we can balance the nitrate ions by inserting a coefficient of 3 in front of the sodium nitrate on the product side.
\[\ce{Fe(NO_3)_3} + 3 \ce{NaOH} \rightarrow \ce{Fe(OH)_3} + 3 \ce{NaNO_3} \nonumber\]
Counting the number of each type of atom on the two sides of the equation will now show that this equation is balanced.
Example \(\PageIndex{2}\)
Write a balanced equation for the reaction that occurs between chlorine gas and aqueous sodium bromide to produce liquid bromine and aqueous sodium chloride.
Solution:
Step 1: Write the word equation (keeping in mind that chlorine and bromine refer to the diatomic molecules).
\[\text{Chlorine} + \text{sodium bromide} \rightarrow \text{bromine} + \text{sodium chloride} \nonumber\]
Step 2: Substitute the correct formulas into the equation.
\[\ce{Cl_2} + \ce{NaBr} \rightarrow \ce{Br_2} + \ce{NaCl} \nonumber\]
Step 3: Insert coefficients where necessary to balance the equation. By placing a coefficient of 2 in front of the \(\ce{NaBr}\), we can balance the bromine atoms and by placing a coefficient of 2 in front of the \(\ce{NaCl}\), we can balance the chlorine atoms.
\[\ce{Cl_2} + 2 \ce{NaBr} \rightarrow \ce{Br_2} + 2 \ce{NaCl} \nonumber\]
A final check (always do this) shows that we have the same number of each atom on the two sides of the equation and we do not have a multiple set of coefficients so this equation is properly balanced.
Example \(\PageIndex{3}\)
Write a balanced equation for the reaction between aluminum sulfate and calcium bromide to produce aluminum bromide and calcium sulfate. (You may need to refer to a chart of polyatomic ions.)
Solution
Step 1: Write the word equation.
\[\text{Aluminum sulfate} + \text{calcium bromide} \rightarrow \text{aluminum bromide} + \text{calcium sulfate} \nonumber\]
Step 2: Replace the names of the substances in the word equation with formulas.
\[\ce{Al_2(SO_4)_3} + \ce{CaBr_2} \rightarrow \ce{AlBr_3} + \ce{CaSO_4} \nonumber\]
Step 3: Insert coefficients to balance the equation. In order to balance the aluminum atoms, we must insert a coefficient of 2 in front of the aluminum compound in the products.
\[\ce{Al_2(SO_4)_3} + \ce{CaBr_2} \rightarrow 2 \ce{AlBr_3} + \ce{CaSO_4} \nonumber\]
In order to balance the sulfate ions, we must insert a coefficient of 3 in front of the \(\ce{CaSO_4}\) in the products.
\[\ce{Al_2(SO_4)_3} + \ce{CaBr_2} \rightarrow 2 \ce{AlBr_3} + 3 \ce{CaSO_4} \nonumber\]
In order to balance the bromine atoms, we must insert a coefficient of 3 in front of the \(\ce{CaBr_2}\) in the reactants.
\[\ce{Al_2(SO_4)_3} + 3 \ce{CaBr_2} \rightarrow 2 \ce{AlBr_3} + 3 \ce{CaSO_4} \nonumber\]
The insertion of the 3 in front of the \(\ce{CaBr_2}\) in the reactants also balances the calcium atoms in the \(\ce{CaSO_4}\) in the products. A final check shows 2 aluminum atoms on each side, 3 sulfur atoms on each side, 12 oxygen atoms on each side, 3 calcium atoms on each side, and 6 bromine atoms on each side. This equation is balanced.
Coefficient Ratios
Chemical equations should be balanced with the simplest whole number coefficients that balance the equation. Here is the properly balanced equation from the previous section.
\[\ce{Al_2(SO_4)_3} + 3 \ce{CaBr_2} \rightarrow 2 \ce{AlBr_3} + 3 \ce{CaSO_4}\]
Note that the equation in the previous section would have the same number of atoms of each type on each side of the equation with the following set of coefficients.
\[2 \ce{Al_2(SO_4)_3} + 6 \ce{CaBr_2} \rightarrow 4 \ce{AlBr_3} + 6 \ce{CaSO_4}\]
Count the number of each type of atom on each side of the equation to confirm that this equation is "balanced". While this set of coefficients does "balance" the equation, they are not the lowest set of coefficients possible that balance the equation. We could divide each of the coefficients in this equation by 2 and get another set of coefficients that are whole numbers and also balance the equation. Since it is required that an equation be balanced with the lowest whole number coefficients, the last equation is NOT properly balanced. When you have finished balancing an equation, you should not only check to make sure it is balanced, you should also check to make sure that it is balanced with the simplest set of whole number coefficients possible.
Example \(\PageIndex{4}\)
Balance each of the following reactions.
- \(\ce{CaCO_3} \left( s \right) \rightarrow \ce{CaO} \left( s \right) + \ce{CO_2} \left( g \right)\)
- \(\ce{H_2SO_4} \left( aq \right) + \ce{Al(OH)_3} \left( aq \right) \rightarrow \ce{Al_2(SO_4)_3} \left( aq \right) + \ce{H_2O} \left( l \right)\)
- \(\ce{Ba(NO_3)_2} \left( aq \right) + \ce{Na_2CO_3} \left( aq \right) \rightarrow \ce{BaCO_3} \left( aq \right) + \ce{NaNO_3} \left( aq \right)\)
- \(\ce{C_2H_4} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right)\)
Solution
- \(\ce{CaCO_3} \left( s \right) \rightarrow \ce{CaO} \left( s \right) + \ce{CO_2} \left( g \right)\) (In this case, the equation balances with all coefficients being 1)
- \(3 \ce{H_2SO_4} \left( aq \right) + 2 \ce{Al(OH)_3} \left( aq \right) \rightarrow \ce{Al_2(SO_4)_3} \left( aq \right) + 6 \ce{H_2O} \left( l \right)\)
- \(\ce{Ba(NO_3)_2} \left( aq \right) + \ce{Na_2CO_3} \left( aq \right) \rightarrow \ce{BaCO_3} \left( aq \right) + 2 \ce{NaNO_3} \left( aq \right)\)
- \(\ce{C_2H_4} \left( g \right) + 3 \ce{O_2} \left( g \right) \rightarrow 2 \ce{CO_2} \left( g \right) + 2 \ce{H_2O} \left( l \right)\)
Conservation of Mass in Chemical Reactions
In Chapter 2 we discussed the development of the atomic theory, or the idea that everything is made of atoms. A strong piece of evidence for this theory was experimentally determined by Antoine Lavoisier, a French chemist. The Law of Conservation of Mass, as he states it, says that mass is conserved in chemical reactions. In other words, the mass of the starting materials (reactants) is always equal to the mass of the ending materials (products).
But what does this really mean? Dalton used this finding to support the idea of atoms. If the mass isn't changing, then the particles that carry the mass (atoms) aren't created or destroyed, but are only rearranged in a chemical reaction. Both the numbers of each type of atom and the mass are conserved during chemical reactions. An examination of a properly balanced equation will demonstrate that mass is conserved. Consider the following reaction.
\[\ce{Fe(NO_3)_3} + 3 \ce{NaOH} \rightarrow \ce{Fe(OH)_3} + 3 \ce{NaNO_3}\]
You should check that this equation is balanced by counting the number of each type of atom on each side of the equation.
We can also determine that mass is conserved in this reaction by determining the total mass on the two sides of the equation. We will use the molar masses to add up the masses of the atoms on the reactant side and compare this to the mass of the atoms on the product side of the reaction:
Reactant Side Mass
1 mole of \(\ce{Fe(NO_3)_3} \: \times\) molar mass \(= \left( 1 \: \text{mol} \right) \left( 241.9 \: \text{g/mol} \right) = 241.9 \: \text{g}\)
3 moles of \(\ce{NaOH} \: \times\) molar mass \(= \left( 3 \: \text{mol} \right) \left( 40.0 \: \text{g/mol} \right) = 120.0 \: \text{g}\)
Total mass for reactants \(= 241.9 \: \text{g} + 120.0 \: \text{g} = 261.9 \: \text{g}\)
Product Side Mass
1 mole of \(\ce{Fe(OH)_3} \: \times\) molar mass \(= \left( 1 \: \text{mol} \right) \left( 106.9 \: \text{g} \right) = 106.9 \: \text{g}\)
3 moles of \(\ce{NaNO_3} \: \times\) molar mass \(= \left( 3 \: \text{mol} \right) \left( 85.0 \: \text{g} \right) = 255.0 \: \text{g}\)
Total mass for products \(= 106.9 \: \text{g} + 255.0 \: \text{g} = 361.9 \: \text{g}\)
As you can see, both number of atom types and mass are conserved during chemical reactions. A group of 20 objects stacked in different ways will still have the same total mass no matter how you stack them.
Video: The law of Conservation of Mass
Summary
A chemical reaction is the process in which one or more substances are changed into one or more new substances. Chemical reactions are represented by chemical equations. Chemical equations have reactants on the left, an arrow that is read as "yields", and the products on the right. To be useful, chemical equations must always be balanced. Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must be the simplest whole number ratio. Mass is always conserved in chemical reactions.
Vocabulary
- Chemical reaction: The process in which one or more substances are changed into one or more new substances.
- Reactants: The starting materials in a reaction.
- Products: Materials present at the end of a reaction.
- Balanced chemical equation: A chemical equation in which the number of each type of atom is equal on the two sides of the equation.
- Subscripts: Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element.
- Coefficient: A small whole number that appears in front of a formula in a balanced chemical equation.

