4.2: Types of Compounds and Their Properties
- Page ID
- 49077
Skills to Develop
- Distinguish between ionic, covalent, and metallic bonding in terms of electron behavior.
- Given a formula, classify a compound as ionic, covalent, or metallic.
- List properties of ionic, covalent, and metallic compounds.
Before students begin the study of chemistry, they usually think that the most stable form for an element is that of a neutral atom. As it happens, that particular idea is not true. If we were to consider the amount of sodium in the earth, we would find a rather large amount, approximately 190,000,000,000,000,000 kilotons. How much of this sodium would we find in the elemental form of sodium atoms? The answer is almost none. The only sodium metal that exists in the earth in the elemental form is that which has been man-made and is kept in chemistry labs and storerooms. Because sodium reacts readily with oxygen in the air and reacts explosively with water, it must be stored in chemistry storerooms under kerosene or mineral oil to keep it away from air and water. If those \(1.9 \times 10^{17}\) kilotons of sodium are not in the form of atoms, in what form are they? Virtually all the sodium in the earth is in the form of sodium ions, \(\ce{Na}^+\).
If all those tons of sodium ion can be found in nature and no sodium atoms can be found, it seems reasonable to suggest that, at least in the case of sodium, the ions are chemically more stable than the atoms. By chemically stable, we mean less likely to undergo chemical change. This is true not only for sodium but for many other elements as well.
Octet Rule
Recall that the noble gas elements are the least reactive of all the elements on the periodic table - they almost never form any type of compound. Their electron configuration is the most stable of all of the elements, having their \(s\) and \(p\) sublevels filled. The noble gases have what is frequently referred to as an "octet", meaning they have eight valence electrons. The other elements are typically more stable if they have an octet, too. Other atoms will gain electrons, lose electrons, or share electrons in order to obtain an octet. The way in which an atom gets an octet determines the type of bond formed.
When an atom gains electrons, the atom will obtain a negative charge and is now called an anion. When an atom loses electrons, the atom will obtain a positive charge and is now called a cation. This may feel backwards, but remember that electrons themselves have a negative charge. When anions and cations are bonded together, the bond is said to be ionic. Metal atoms will lose electrons to obtain an octet and nonmetals will gain electrons. Therefore, in an ionic bond, metals are typically bonded to nonmetals.
Some atoms are capable of obtaining an octet by sharing their valence electrons with another atom. This type of bonding is called a covalent bond. Only nonmetals are capable of forming covalent bonds with other nonmetals.
Properties of Ionic Compounds
When ionic compounds are formed, we are almost never dealing with just a single positive ion and a single negative ion. When ionic compounds are formed in laboratory conditions, many cations and anions are formed at the same time. The positive and negative ions are not just attracted to a single oppositely charged ion. The ions are attracted to several of the oppositely charged ions. The ions arrange themselves into organized patterns where each ion is surrounded by several ions of the opposite charge. The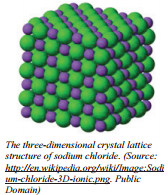 organized patterns of positive and negative ions are called lattice structures. Because ionic compounds form these larger lattice structures in the solid phase, they are not referred to as "molecules" but rather as lattice structures or crystals.
organized patterns of positive and negative ions are called lattice structures. Because ionic compounds form these larger lattice structures in the solid phase, they are not referred to as "molecules" but rather as lattice structures or crystals.
The image shows the solid structure of sodium chloride. Each sodium ion is touching six chloride ions - the four surrounding ones and the one above and one below. Each chloride ion is touching six sodium ions in the same way.
When electrons are transferred from metallic atoms to nonmetallic atoms during the formation of an ionic bond, the electron transfer is permanent. That is, the electrons now belong to the nonmetallic ion. This compound does not act like sodium and chlorine atoms did before they combined. This compound will act as sodium cations and chloride anions. If the compound is melted or dissolved, the particles come apart in the form of ions.
 The electrostatic attraction between the oppositely charged ions is quite strong and therefore, ionic compounds have very high melting points. Sodium chloride (table salt), for example, must be heated to around \(800^\text{o}C\) to melt and around \(1500^\text{o}C\) to boil. There is only one type of solid that has higher melting points and boiling points, in general, than ionic compounds.
The electrostatic attraction between the oppositely charged ions is quite strong and therefore, ionic compounds have very high melting points. Sodium chloride (table salt), for example, must be heated to around \(800^\text{o}C\) to melt and around \(1500^\text{o}C\) to boil. There is only one type of solid that has higher melting points and boiling points, in general, than ionic compounds.
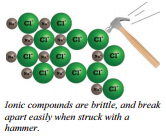
You can see that negative ions are surrounded by positive ions and vice versa. If part of the lattice is pushed downward, negative ions will then be next to negative ions and the structure will break up; therefore, ionic compounds tend to be brittle solids. If you attempt to hammer on ionic substances, they will shatter. This is very different from metals which can be hammered into different shapes without the metal atoms separating from each other.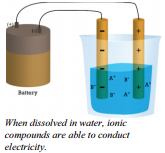
Ionic substances generally dissolve readily in water. In an ionic compound that has been melted or an ionic compound dissolved in water, ions are present that have the ability to move around in the liquid. The presence of the mobile ions in liquid or solution allow the solution to conduct electric current.
Properties of Covalent Compounds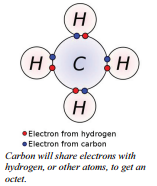
In ionic compounds, we learned that atoms are able to achieve an octet through a metal giving away electrons (forming cations) and nonmetals taking electrons (forming anions). Some elements, however, can achieve an octet a different way, by sharing their valence electrons with other atoms instead. Typically, only nonmetals and sometimes metalloids are able to form covalent bonds. Metals, with their low numbers of valence electrons, are unable to achieve an octet through sharing valence electrons.
The term covalent bond dates from 1939. The prefix co- means jointly (as in, coworker, cooperate, etc.), "valent" is referring to an atom's valence electrons. thus, a "co-valent bond," essentially, means that the atoms share valence electrons.
Covalent compounds have properties very different from ionic compounds. Ionic compounds have high melting points causing them to be solid at room temperature, and conduct electricity when dissolved in water. Covalent compounds have low melting points and many are liquids or gases at room temperature. Whereas most ionic compounds are capable of dissolving in water, many covalent compounds do not. Also unlike ionic compounds, when covalent compounds are dissolved in water, they are not conductors of electricity.
Properties of Metallic Bonds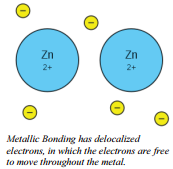
There is a third type of bond that may be formed between two atoms. In metallic bonding, the electrons between neighboring metal atoms are delocalized, meaning that the electrons are not tied to one atom specifically. The electrons, instead, are gathered in what we call an "electron sea." In an electron sea, the metal nuclei form the basis, and the electrons move around the nuclei.
Because of this unique type of bonding structure, metallic bonding accounts for many physical properties of metals, such as strength, malleability (or bendability), ductility, conductivity (allows heat and electricity to go through), and luster (shine).
Properties of Compounds vs. the Elements of which they are composed
It is important to point out that when elements combine to form covalent compounds or ionic compounds the properties of the compound are different from the properties of the elements from which the compound is formed. Consider, for example, sugar, formed from the elements carbon, hydrogen, and oxygen: \(\ce{C_{12}H_{22}O_{11}}\). Forms of carbon you are probably familiar with include coal and graphite (pencil lead). Oxygen is a gas necessary for your survival, and hydrogen is also a very flammable gas. You wouldn't want the sugar you put on your cereal to taste like coal or be as flammable as hydrogen and oxygen gases. When combined, a new compound is made with its own unique properties, different from the elements that formed the compound.
The process of gaining and/or losing electrons completely changes the chemical properties of the substances. The chemical and physical properties of an ionic compound will bear no resemblance to the properties of the elements which formed the ions. For example, sodium is a metal that is shiny, an excellent conductor of electric current, and reacts violently with water. Chlorine is a poisonous gas. When sodium and chlorine are chemically combined to form sodium chloride (table salt), the product has an entirely new set of properties. Sometimes, we sprinkle sodium chloride on our food. This is not something we would do if we expected it to explode when contacted by water or if we expected it to poison us.
What happens when these elements combine in different ratios, forming compounds such as isopropyl alcohol (commonly called rubbing alcohol), \(\ce{C_3H_7_OH}\), or acetone (the main ingredient in most finger nail polish removers), \(\ce{C_3H_6O}\)? Does rubbing alcohol have the same properties has finger nail polish remover? No! When elements combine in different ratios, different compounds are formed which have their own unique properties. Each compound will typically have its own melting point, boiling point, and density. Frequently, they will have a unique smell or taste. They will also have unique chemical properties and react differently from other compounds.
Lesson Summary
- The octet rule is an expression of the tendency for atoms to gain or lose the appropriate number of electrons so that the resulting ion has either completely filled or completely emptied outer energy levels.
- Ionic compounds form ionic crystal lattices rather than molecules, have very high melting and boiling points, and tend to be brittle solids. They are generally soluble in water and their water solutions will conduct electricity.
- Covalent compounds are formed from nonmetals sharing electrons. They tend to have low melting and boiling points. Although some are soluble in water, they do not conduct electricity when dissolved.
- Metallic bonds allow the electrons to move freely, resulting in materials that are very conductive, malleable, and lustrous.
- Compounds have chemical properties that are unrelated to the chemical properties of the elements from which they were formed.
Vocabulary
- Octet Rule: The tendency of an atom to be more stable with eight valence electrons.
- Ionic compound: A positively charged particle (typically a metal) bonded to a negatively charged particle (typically a nonmetal) held together by electrostatic attraction.
- Covalent compound: Two or more atoms (typically nonmetals) forming a molecule in which electrons are being shared between atoms.
Further Reading/Supplementary Links
- To see a video and clips discussing the types of bonds, go to http://www.uen.org/dms/. Go to the k-12 library. Search for covalent bond, metallic bond, or ionic bond.

