4.1: Introduction to Compounds
- Page ID
- 49076
Skills to Develop
- Explain the difference between an element, a compound, and a mixture.
Substances and Mixtures
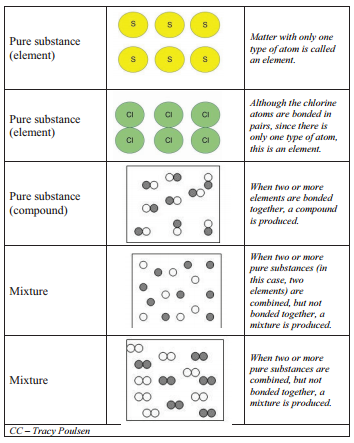
Matter can be classified into two broad categories: pure substances and mixtures. A pure substance is a form of matter that has a constant composition (meaning it's the same everywhere) and properties that are constant throughout the sample (meaning there is only one set of properties such as melting point, color, boiling point, etc. throughout the matter). Elements and compounds are both examples of pure substances.
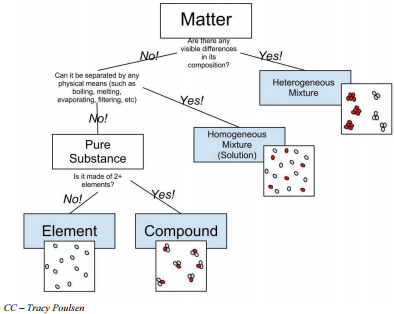
Mixtures are physical combinations of two or more elements and/or compounds. The term "physical combination" refers to mixing two different substances together where the substances do not chemically react. The physical appearance of the substances may change but the atoms and/or molecules in the substance do not change.
The chemical symbols are used not only to represent the elements; they are also used to write chemical formulas for the millions of compounds formed when elements chemically combine to form compounds. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The law of constant composition can be used to distinguish between compounds and mixtures. Compounds have a constant composition, and mixtures do not. For example, pure water is always \(88.8\%\) oxygen and \(11.2\%\) hydrogen by weight, regardless of the source of the water. Because water is a compound, it will always have this exact composition. Brass is an example of a mixture. Brass consists of two elements, copper and zinc, but it can contain as little as \(10\%\) or as much as \(45\%\) zinc.
Consider the following examples including elements, compounds, and mixtures
The last couple of chapters have focused on elements and their properties. This unit will focus on compounds, including what compounds form and how elements combine to make compounds. Later chapters will cover mixtures.
Compounds and Chemical Formulas
The formula for a compound uses the symbols to indicate the type of atoms involved and uses subscripts to indicate the number of each atom in the formula. For example, aluminum combines with oxygen to form the compound aluminum oxide. To form aluminum oxide requires two atoms of aluminum and three atoms of oxygen. Therefore, we write the formula for aluminum oxide as \(\ce{Al_2O_3}\). The symbol \(\ce{Al}\) tells us that the compound contains aluminum, and the subscript 2 tells us that there are two atoms of aluminum in each molecule. the \(\ce{O}\) tells us that the compound contains oxygen, and the subscript 3 tells us that there are three atoms of oxygen in each molecule. It was decided by chemists that when the subscript for an element is 1, no subscript would be used at all. Thus the chemical formula \(\ce{MgCl_2}\) tells us that one molecule of this substance contains one atom of magnesium and two atoms of chlorine. In formulas that contain parentheses, such as \(\ce{Ca(OH)_2}\), the subscript 2 applies to everything inside the parentheses. Therefore, this formula (calcium hydroxide) contains one atom of calcium and two atoms of oxygen and two atoms of hydrogen.
Lesson Summary
- Matter can be classified into two broad categories: pure substances and mixtures.
- A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample.
- Mixtures are physical combinations of two or more elements and/or compounds.
- Elements and compounds are both examples of pure substances.
- Compounds are substances that are made up of more than one type of atom.
- Elements are the simplest substances made up of only one type of atom.
Vocabulary
- Element: A substance that is made up of only one type of atom.
- Compound: A substance that is made up of more than one type of atom bonded together.
- Mixture: A combination of two or more elements or compounds which have not reacted to bond together; each part in the mixture retains its own properties.
Further Reading/Supplementary Links
- You may listen to Tom Lehrer's humorous song "The Elements" with animation at The Element Song (http://www.privatehand.com/flash/elements.html)
- The learner.org website allows users to view streaming videos of the Annenberg series of chemistry videos. You are required to register before you can watch the videos but there is no charge. Videos on Demand - The World of Chemistry (http://www.learner.org/resources/ser...p=yes&pid=793#)

