3.5: Periodic Trends
- Page ID
- 49074
Skills to Develop
- Explain what is meant by the term periodic law.
- Describe the general trend in atomic size for groups and periods.
- Describe the trends that exist in the periodic table for ionization energy.
- Describe the trends that exist in the periodic table for electronegativity.
We have talked in great detail about how the periodic table was developed, but we have yet to talk about where the periodic table gets its name. To be periodic means to "have repeating cycles" or repeating patterns. In the periodic table, there are a number of physical properties that are "trend-like". This means that as you move down a group or across a period, you will see the properties changing in a general direction.
The periodic table is a powerful tool that provides a way for chemists to organize the chemical elements. The word "periodic" means happening or recurring at regular intervals. The periodic law states that the properties of the elements recur periodically as their atomic numbers increase. The electron configurations of the atoms vary periodically with their atomic number. Because the physical and chemical properties of elements depend on their electron configurations, many of the physical and chemical properties of the elements tend to repeat in a pattern.
The actual repeating trends that are observed have to do with three factors. These factors are:
- The number of protons in the nucleus (called the nuclear charge).
- The number of energy levels holding electrons (and the number of electrons in the outer energy level).
- The number of electrons held between the nucleus and its outermost electrons (called the shielding effect). This affects the attraction between the valence electrons and the protons in the nucleus.
Trends in Atomic Radius
The atomic radius is a way of measuring the size of an atom. Although this is difficult to directly measure, we are, in essence, looking at the distance from the nucleus to the outermost electrons.
Let's look at the atomic radii or the size of the atom from the top of a family or group to the bottom. Take, for example, the Group 1 metals. Each atom in this family (and all other main group families) has the same number of electrons in the outer energy level as all the other atoms of that family. Each row (period) in the periodic table represents another added energy level. When we first learned about principal energy levels, we learned that each new energy level was larger than the one before. Energy level 2 is larger than energy level 1, energy level 3 is larger than energy level 2, and so on. Therefore, as we move down the periodic table from period to period, each successive period represents the addition of a larger energy level.
You can imagine that with the increase in the number of energy levels, the size of the atom must increase. The increase in the number of energy levels in the electron cloud takes up more space. Therefore the trend within a group or family on the periodic table is that the atomic size increases with increased number of energy levels.
In order to determine the trend for the periods, we need to look at the number of protons (nuclear charge), the number of energy levels, and the shielding effect. For a row in the periodic table, the atomic number still increases (as it did for the groups) and thus the number of protons would increase. When we examine the energy levels for period 2, we find that the outermost energy level does not change as we increase the number of electrons. In period 2, each additional electron goes into the second energy level. So the number of energy levels does not go up. As we move from left to right across a period, the number of electrons in the outer energy level increases but it is the same outer energy level.
 Looking at the elements in period 2, the number of protons increases from lithium with three protons, to fluorine with nine protons. Therefore, the nuclear charge increases across a period. Meanwhile, the number of energy levels occupied by electrons remains the same. The numbers of electrons in the outermost energy level increases from left to right across a period, but how will this affect the radius? With an increase in nuclear charge, there is an increase in the pull between protons and the outer level, pulling the outer electrons toward the nucleus. The net result is that the atomic size decreases going across the row.
Looking at the elements in period 2, the number of protons increases from lithium with three protons, to fluorine with nine protons. Therefore, the nuclear charge increases across a period. Meanwhile, the number of energy levels occupied by electrons remains the same. The numbers of electrons in the outermost energy level increases from left to right across a period, but how will this affect the radius? With an increase in nuclear charge, there is an increase in the pull between protons and the outer level, pulling the outer electrons toward the nucleus. The net result is that the atomic size decreases going across the row.
Considering all the information about atomic size, you will recognize that the largest atom on the periodic table is all the way to the left and all the way to the bottom, francium, #87, and the smallest atom is all the way to the right and all the way to the top, helium, #2.
The fact that the atoms get larger as you move downward in a family is probably exactly what you expected before you even read this section, but the fact that the atoms get smaller as you move to the right across a period is most likely a big surprise. Make sure you understand this trend and the reasons for it.
Example
Which of the following has a greater radius?
- \(\ce{As}\) or \(\ce{Sb}\)
- \(\ce{Ca}\) or \(\ce{K}\)
- Polonium or Sulfur
Solution
- \(\ce{Sb}\) because it is below \(\ce{As}\) in Group 15.
- \(\ce{K}\) because it is further to the left on the periodic table.
- Polonium because it is below Sulfur in Group 16.
Periodic Trends in Ionization Energy
Lithium as an electron configuration of \(1s^2 2s^1\). Lithium has one electron in its outermost energy level. In order to remove this electron, energy must be added. Look at the equation below:
\[\ce{Li} \left( g \right) + \text{energy} \rightarrow \ce{Li}^+ \left( g \right) + e^-\]
With the addition of energy, a lithium ion can be formed from the lithium atom by losing one electron. This energy is known as the ionization energy. The ionization energy is the energy required to remove the most loosely held electron from a gaseous atom. The higher the value of the ionization energy, the harder it is to remove that electron.
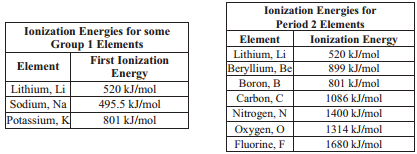
Consider the ionization energies for the elements in group 1A of the periodic table, the alkali metals. Comparing the electron configuration of lithium to potassium, we know that the electron to be removed is further away from the nucleus, as the energy level of the valence electron increases. Because potassium's valence electron is further from the nucleus, there is less attraction between this electron and the protons and it requires less energy to remove this electron. As you move down a family (or group) on the periodic table, the ionization energy decreases.
We can see a trend when we look at the ionization energies for the elements in period 2. When we look closely at the data presented in the table above, we can see that as we move across the period from left to right, in general, the ionization energy increases. As we move across the period, the atoms become smaller which causes the nucleus to have greater attraction for the valence electrons. Therefore, as you move from left to right in a period on the periodic table, the ionization energy increases.
Example
Which of the following has a greater ionization energy?
- \(\ce{As}\) or \(\ce{Sb}\)
- \(\ce{Ca}\) or \(\ce{K}\)
- Polonium or Sulfur
Solution:
- \(\ce{As}\) because it is above \(\ce{Sb}\) in Group 15.
- \(\ce{Ca}\) because it is further to the right on the periodic table.
- \(\ce{S}\) because it is above \(\ce{Po}\) in Group 16.
Periodic Trends in Electronegativity
Around 1935, the American chemist Linus Pauling developed a scale to describe the attraction an element has for electrons in a chemical bond. This is the electronegativity. The values of electronegativity are higher for elements that more strongly attract electrons. On this Pauling scale fluorine, with an electronegativity of 4.0, is the most electronegative element, and cesium and francium, with electronegativities of 0.7, are the least electronegative.
The electronegativity of atoms increases as you move from left to right across a period in the periodic table. This is because as you go from left to right across a period, the atoms of each element have the same number of energy levels. However, the nuclear charge increases, so the attraction that atoms have for the valence electrons increases.
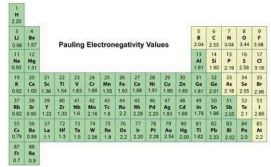 The electronegativity of atoms decreases as you move from top to bottom down a group in the periodic table. This is because as you go from top to bottom down a group, the atoms of each element have an increasing number of energy levels.
The electronegativity of atoms decreases as you move from top to bottom down a group in the periodic table. This is because as you go from top to bottom down a group, the atoms of each element have an increasing number of energy levels.
Atoms with low ionization energies have low electronegativities because their nuclei do not have a strong attraction for electrons. Atoms with high ionization energies have high electronegativities because the nucleus has a strong attraction for electrons.
Lesson Summary
- Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located.
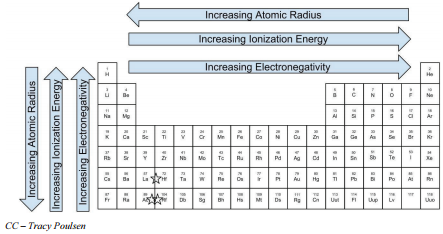
- The atomic radius increases from the top to the bottom in any group and decreases from left to right across a period.
- Ionization energy is the energy required to remove the most loosely held electron from a gaseous atom or ion.
- Ionization energy generally increases across a period and decreases down a group.
- The higher the electronegativity of an atom, the greater its ability to attract shared electrons.
- The electronegativity of atoms increases as you move from left to right across a period in the periodic table and decreases as you move from top to bottom down a group in the periodic table.
Vocabulary
- Nuclear charge: The number of protons in the nucleus.
- Shielding effect: The inner electrons help "shield" the outer electrons and the nucleus from each other.
- Ionization energy: The energy required to remove the most loosely held electron from a gaseous atom or ion.
- Electronegativity: The ability of an atom in a molecule to attract shared electrons.

