2.7: Electron Arrangement in Atoms
- Page ID
- 49039
Skills to Develop
- List the order in which electron energy levels/sublevels will fill.
- Write the electron configuration and abbreviated electron configuration for a given atom.
Chemists are particularly interested in the electrons in an atom's electron cloud. This is because the electrons determine the chemical properties of elements, such as what compounds the element will form and which reactions it will participate in. In this section, we will learn where the electrons are in atoms.
Electron Energy Levels
Although Bohr's model was particularly useful for hydrogen, it did not work well for elements larger than hydrogen. However, other physicists built on his model to create one that worked for all elements. It was found that the energy levels used for hydrogen were further composed of sublevels of different shapes. These sublevels were composed of orbitals in which the electrons were located.
The following table summarizes the possible energy levels and sublevels, including the number of orbitals that compose each sublevel and the number of electrons the sublevel can hold when completely filled.
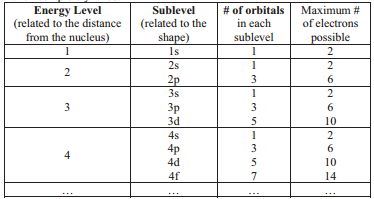
There are several patterns to notice when looking at the table of energy levels. Each energy level has one more sublevel than the level before it. Also, each new sublevel has two more orbitals. Can you predict what the 5\(^\text{th}\) energy level would look like?
When determining where the electrons in an atom are located, a couple of rules must be followed:
1. Each added electron enters the orbitals of the lowest energy available.
2. No more than two electrons can be placed in any orbital.
The Electron Configuration
It would be convenient if the sublevels filled in the order listed in the table, such as \(1s\), \(2s\), \(2p\), \(3s\), \(3p\), \(3d\), \(4s\), \(4p\), etc. However, this is not the order the electrons fill the sublevels. Remember, the electrons will always go to the lowest energy available. When that is taken into account, the actual filling order is:
\(1s\), \(2s\), \(2p\), \(3s\), \(3p\), \(4s\), \(3d\), \(4p\), \(5s\), \(4d\), \(5p\), \(6s\), \(4f\), \(5d\), \(6p\), \(7s\), \(5f\), \(6d\), \(7p\). . .
Note that \(4s\) has lower energy than \(3d\) and, therefore, will fill first. The filling order gets more overlapped the higher you go.
An electron configuration lists the number of electrons in each used sublevel for an atom. For example, consider the element gallium, with 31 electrons. Its first two electrons would fit in the lowest energy possible, \(1s\). The next two would occupy \(2s\). \(2p\), with three orbitals, can hold its next 6 electrons. Gallium continues to fill up its orbitals, finally putting 1 electron in \(4p\). The electron configuration for gallium would be:
\[_{31}\ce{Ga}: \: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^1\]
Although you can choose to memorize the list and how many electrons fit in each sublevel for the purpose of writing electron configurations, there is a way for us to find this order by simply using our periodic table.
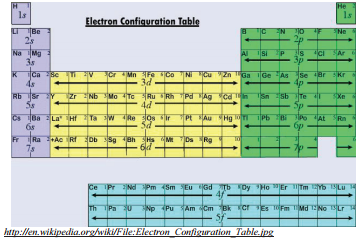
Look at the different sections of the periodic table. You may have noticed that there are several natural sections of the periodic table. The first 2 columns on the left make up the first section; the six columns on the right make up the next section; the middle ten columns make up another section; finally, the bottom fourteen columns compose the last section. Note the significance of these numbers: 2 electrons fit in any \(s\) sublevel, 6 electrons fit in any \(p\) sublevel, 10 electrons fit in any \(d\) sublevel, and 14 electrons fit in any \(f\) sublevel. The four sections described previously are known as the \(s\), \(p\), \(d\), and \(f\) blocks respectively.
If you move across the rows starting at the top left of the periodic table and move across each successive row, you can generate the same order of filling orbitals that was listed before and also how many electrons total fit in each orbital. Starting at the top left, you are filling \(1s\). Moving onto the second row, \(2s\) is filled followed by \(2p\). Continuing with the filling order you generate the list:
\(1s\), \(2s\), \(2p\), \(3s\), \(3p\), \(4s\), \(3d\), \(4p\), \(5s\), \(4d\), \(5p\), \(6s\), \(4f\), \(5d\), \(6p\), \(7s\), \(5f\), \(6d\), \(7p\). . .
An electron configuration lists the sublevels the electrons occupy and the number of electrons in each of those sublevels, written as superscripts.
Example 2.7.1
Write the electron configurations for
- Potassium, \(\ce{K}\)
- Arsenic, \(\ce{As}\)
- Phosphorus, \(\ce{P}\)
Solution
a) Potassium atoms have 19 protons and, therefore, 19 electrons. Using our chart, we see that the first sublevel to fill is \(1s\), which can hold 2 of those 19 electrons. Next to fill is \(2s\), which also holds 2 electrons. Then comes \(2p\), which holds 6 electrons. We keep filling up the sublevels until all 19 of the electrons have been placed in the lowest energy level possible. \(4s\) only has 1 electron in it, although it can hold up to 2 electrons, because there are only 19 electrons total in potassium. Its electron configuration is written as: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^1\)
b) \(_{33}\ce{As}: \: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^3\)
c) \(_{15}\ce{P}: \: 1s^2 2s^2 2p^6 3s^2 3p^3\)
Abbreviated Electron Configuration
As the electron configurations become longer and longer, it becomes tedious to write them out. A shortcut has been devised so that writing configurations is less tedious. The electron configuration for potassium is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^1\). The electron configuration for potassium is the same as the electron configuration for argon except that it has one more electron. The electron configuration for argon is \(1s^2 2s^2 2p^6 3s^2 3p^6\) and in order to write the electron configuration for potassium, we need to add only \(4s^1\). It is acceptable to use \(\left[ Ar \right]\) to represent the electron configuration for argon and \(\left[ Ar \right] 4s^1\) to represent the electron configuration for potassium. Using this shortcut, the abbreviated electron configuration for calcium would be \(\left[ Ar \right] 4s^2\) and the electron configuration for scandium would be \(\left[ Ar \right] 4s^2 3d^1\).
Even though the periodic table was organized according to the chemical behavior of the elements, you can now see that the shape and design of the table is a perfect reflection of the electron configuration of the atoms. This is because the chemical behavior of the elements is also caused by the electron configuration of the atoms.
Example 2.7.2
Write the abbreviated electron configurations for
- Potassium, \(\ce{K}\)
- Arsenic, \(\ce{As}\)
- Phosphorus, \(\ce{P}\)
Solution
- \(_{19}\ce{K}: \: \left[ Ar \right] 4s^1\)
- \(_{33}\ce{As}: \: \left[ Ar \right] 4s^2 3d^{10} 4p^3\)
- \(_{15}\ce{P}: \: \left[ Ne \right] 3s^2 3p^3\)
Summary
- Electrons are located in orbitals, in various sublevels and energy levels of atoms.
- Electrons will occupy the lowest energy level possible.
- It is possible to write the electron configuration of an element using a periodic table.
Vocabulary
- Electron configuration: A list that represents the arrangement of electrons in an atom.

