16: Homework Solutions
- Page ID
- 43950
Amino Acids
(01)
- non-polar
- basic
- non-polar
- non-polar
- acidic
(02)
- tryptophan (Trp); phenylalanine (Phe); tyrosine (Tyr)
- serine (Ser); Threonine (Thr)
- glutamine (Gln); asparagine (Asn)
- tryptophan (Try); lysine (Lys); Arginine (Arg); Histidine (His)
- tyrosine (Tyr)
(03) Thiols contain a sulfur atom bonded to hydrogen (the sulfur analog of an alcohol). There is one amino acid, cysteine (Cys), that contains a thiol. Note that methione is a sulfide the sulfur analog of an ether.
(04)

- See above. The amine is ionic because it is protonated at physiological pH.
- See above. Carboxylates are ionized carboxylic acids that have lost their acidic proton.
- zwitterion: a neutral dipolar ion that has one + charge and one - charge; Asp, Glu, Lys, Arg, His
- See above. glutamine (Glu)
- polar

(05) Fill in the following table with ‘Y’ to indicate ‘yes’ and ‘N’ to indicate ‘no’ for each box as it pertains to each listed amino acid in each category. Some of the names are given only as their three-letter abbreviations.
| Amino Acid | Nonpolar | Polar | Acidic Side Chain | Basic Side Chain | Essential? |
| Proline | Y | N | N | N | N |
| Cys | N | Y | N | N | N |
| Asp | N | N | Y | N | N |
| Serine | N | Y | N | N | N |
| Gln | N | Y | N | N | N |
Chirality and Amino Acids
(06)
- achiral
- achiral
- chiral
- chiral
- chiral
(07)
- hydroxyl groups (alcohols)
- C4H12O; Both compounds have the same chemical formula and same atom connections.
- No, the compounds have different spatial arrangments.
- Only two of the four groups around the chiral carbon can align at once.
- Yes, this pair of enantiomers are non-superimposable mirror images of each other.
- Enantiomers are compounds with 1 or more chiral carbons that are non-superimposable mirror images of each other.
- Yes, each chiral carbon is bonded to four different groups: a hydrogen atom, a hydroxyl group, a methyl group, and an ethyl group.
(08) Racemic mixture: a 1:1 ratio of a pair of enantiomers. Racemic mixtures are NOT optically active.
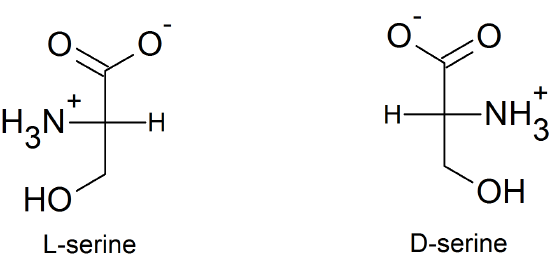
Peptides
(9)
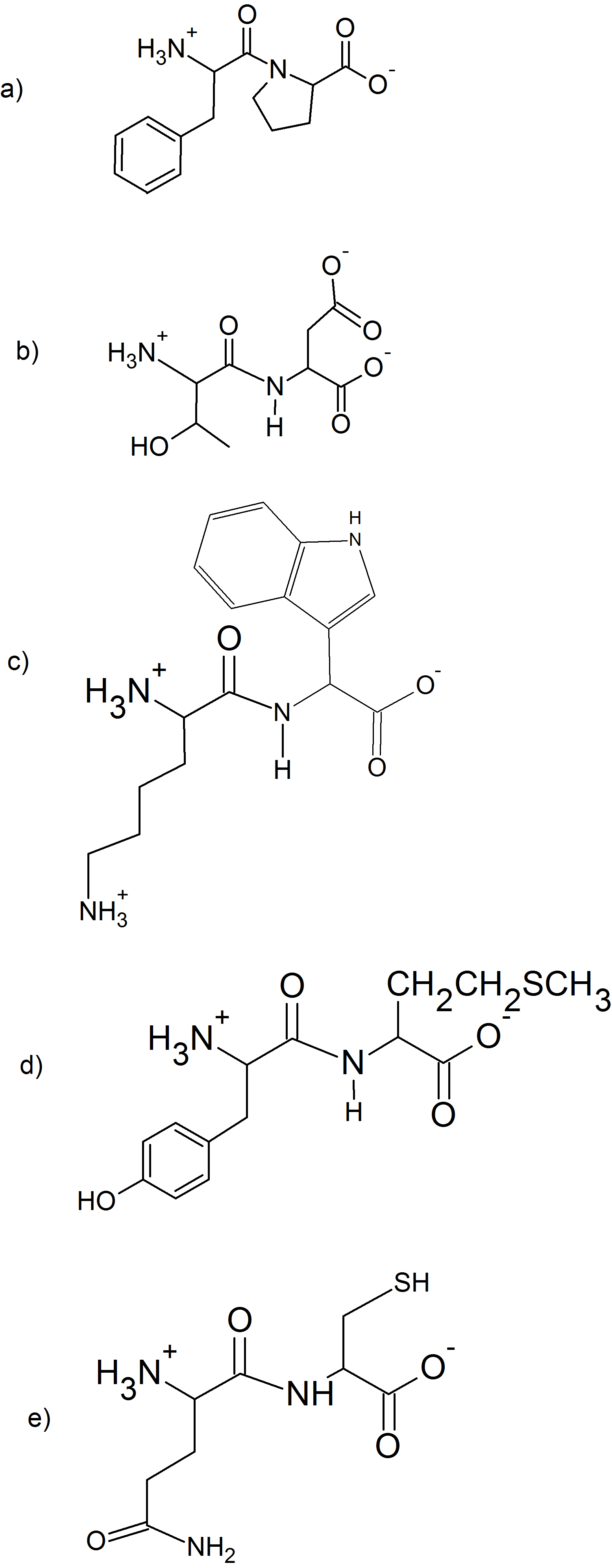
(10)

(11)
- 3 amino acids: Ala-Met-Phe
- N-terminus is Ala and C-terminus is Phe
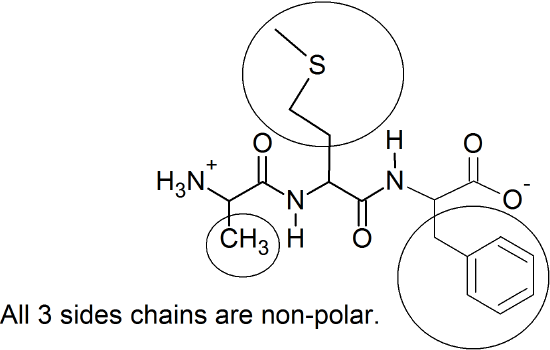
Protein Architecture
(12) disulfide bridges; salt bridges; H-bonding; dispersion forces; (metal-ion coordination is the 5th)
(13) Cysteine (Cys) is the amino acid that can form disulfide bridges.
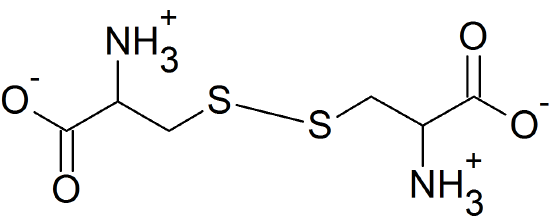
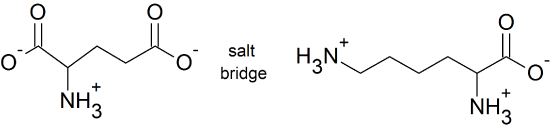
(14) The acidic amino acids, aspartic acid (Asp) & glutamic acid (Glu) and the basic amino acids, lysine (Lys), arginine (Arg), and histidine (His) can form salt bridges because their side chains are ionized. One possible example is shown below using glutamic acid and lysine.
(15) The non-polar regions will be located in the center of a globular protein while the polar regions will be located on the outside of the globule to create water solubility.
(16)
Fibrous proteins are limited to secondary structure and only contain one form - alpha or beta. Fibrous proteins are strong and insoluble in water. Fibrous proteins create structural support. Examples include keratins, elastins, and collagens.
Globular proteins have primary, secondary, tertiary and possibly quaternary structure. They can contain both alpha and beta secondary structures within the same protein. Globular proteins are water soluble and serve as transport compounds (hemoglobin), reaction catalysts (enzymes) and support our immunity (immunoglobins).
Membrane proteins are partially or fully embedded in the cell membrane. They relay signals into or out of the cell or serve are ion channels that facilitate the passage of ions into or out of the cell.
(17)
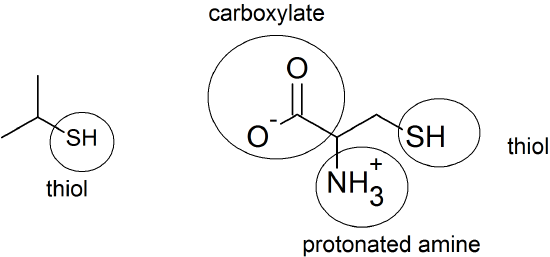
(18) Any three of the following amino acids will repel water via the London dispersion forces: glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), tryptophan (Trp), phenylalanine (Phe), methionine (Met)
(19) Denaturation is the disruption of the secondary, tertiary, and/or quaternary structure of a protein that causes the protein to lose its biological function. Five causes of denaturation are heat changes, physical agitation, addition of detergents, pH changes, and addition of certain metals.
Enzymes
(20) Optimum enzyme conditions for humans are physiological pH (7.4) and 37ºC. Two notable exceptions are enzymes in the stomach (pH 2) and small intestines (pH 8).
(21)
\[ E + S \rightleftharpoons ES \rightleftharpoons E + P\]
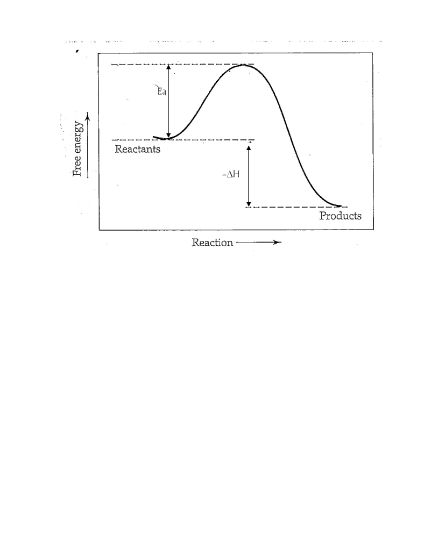
(22)
- no change
- lowers Ea
- increases reaction rate
- no change (The reaction will reach equilibrium faster, but the equilibrium conditions will be the same.)
(23) Determine which of the following enable enzyme-substrate complex formation.
- yes
- yes
- yes
- no
- yes
(24) Most biochemical reactions require a catalyst. The one notable exception is acid-base reactions which happen spontaneously without the presence of a catalyst.
(25) Competitive inhibitors have similar structures, shapes and charge distributions when compared with the substrate so that they can interact with the enzyme at the active site. Non-competitive inhibitors have different structures, shapes and charge distributions when compared withe the substrate and interact with the enzyme at a site DIFFERENT from the active site.
(26)