6: Quantities in Chemical Reactions
- Page ID
- 43920
Molecular Mass, Formula Mass, and Molar Mass
(1) What value is held by Avogadro’s number?
(2) What is the mass of each of the following quantities:
- 1.0 mole of Hydrogen gas
- 2.0 moles of water
- 0.1 moles of lead
- 0.50 mole of hydrochloric acid (HCl)
- 3.0 moles of Helium
(3) Without using a calculator, which has the greater mass: One mole of carbon atoms or one mole of chlorine atoms? Explain your reasoning.
(4) What is the atomic mass of CH3OH, ethanol, in AMU? What is the molar mass?
(5) For each of these different compounds, using the given masses, calculate how many moles each sample contains.
- In 3.1 grams of sodium chloride
- In 4.8 grams of hydrogen peroxide
- In 8 grams of lithium hydroxide
- In 10. grams of oxygen gas
- In 22 grams of ammonia
(6) Contrasting with the previous problem, this time find the mass of each sample by using the given number of moles.
- 6.0 moles of phosphorus
- 4.0 moles of iron
- 5.20 moles of fluorine gas
- 1.3 moles of hydrochloric acid (HCl)
- 7.7 moles of neon
(7) Combine the following ions into an ionic compound and calculate the molar mass.
- a. Ammonium ion and sulfide ion
- b. Strontium ion and nitride ion
- c. Aluminum ion and fluoride ion
- d. Calcium ion and iodide ion
- e. Silver ion and oxide ion
Mole Questions
(8) What is the molar mass of sodium bicarbonate (baking soda)?
(9) How many moles are in a 42 g sample of sodium bicarbonate?
(10) What is the mass in grams of 0.42 moles of sodium bicarbonate?
(11) How many formula units are in a 25 g sample of sodium bicarbonate?
(12) How many sodium ions are in a 3.2g sample of sodium bicarbonate?
(13) What is the molar mass of glycerine (\(C_3H_8O_3\))?
(14) How many moles are in a 42 g sample of glycerine?
(15) What is the mass in grams of 0.42 moles of glycerine?
(16) How many molecules are in a 25 g sample of glycerine?
(17) How many carbon atoms are in a 3.2 g sample of glycerine?
(18) There is a 50 g sample of each of the following substances: \(CH_2O\), \(CH_3CH_2OH\), \(CH_3COCH_3\)
Which sample contains the largest number of molecules?
(19) Answer the following questions for both caffeine (C8H10N4O2) and sodium phosphate.
a) Calculate the molar mass of each.
b) Is each compound covalently or ionically bonded? Explain your reasoning.
c) We have a pure sample of each compound that weighs 2.3 g. How many moles are present in each sample?
(20) There is a 50 g sample of each of the following substances: \(Ca(NO_3)_2\), \(NaHCO_3\), \(CuCl_2\)
a) Which sample contains the largest number of formula units?
b) Which sample contains the largest number of ions?
Writing and Balancing a Chemical Equation
(21) Balance the following chemical equations.
- Al(s) + H2O(l) → Al(OH)3(s) + H2(g)
- \(N_{2\;(g)} + O_{2\; (g)} \rightarrow NO_{2\; (g)}\)
- \(H_2CO_{3\;(aq)} \rightarrow H_2O_{(l)} + CO_{2\; (g)}\)
- \(P_2O_{5\;(s)} \rightarrow P_{4\;(s)} +O_{2\; (g)}\)
- \(N_{2\;(g)} + H_{2\;(g)} + Cl_{2\;(g)} \rightarrow NH_4Cl_{(s)}\)
(22) Balance each of the given combustion reactions.
- \(C_6H_6(l) + O_{2\; (g)} \rightarrow CO_{2\; (g)} + H_2O_{(g)}\)
- \(C_2H_2(g) + O_{2\; (g)} \rightarrow CO_{2\; (g)} + H_2O_{(g)}\)
- \(C_8H_{18}(l) + O_{2\; (g)} \rightarrow CO_{2\; (g)} + H_2O_{(g)}\)
- \(C_4H_9OH(l) + O_{2\; (g)} \rightarrow CO_{2\; (g)} + H_2O_{(g)}\)
- CH3(CH2)4OCH2CH3(l) + O2(g) → CO2(g) + H2O(g)
(23) The next set of chemical equations are NOT properly balanced. Alter the coefficients as needed so that the equations are properly balanced.
- \(N_{2\;(g)} + H_{2\;(g)} \rightarrow 2NH_{3\;(l)} \)
- Ba(OH)2(s) + H2SO4(aq) → BaSO4(s) + H2O(l)
- \(2CH_{4\; (g)} + N_{2\;(g)} \rightarrow HCN(g) + 4H_{2\;(g)} \)
- \(8Cl_{2\;(g)} + 8H_2O(l) \rightarrow 2HClO_{4\;(aq)} + 14HCl(aq) \)
- \(2PH_3(aq) + 2O_{2\; (g)} \rightarrow 2H_3PO_{4\;(aq)} \)
Energy and Chemical Reactions
(24) Follow the directions in each of the following sets of conversions.
- Convert 6.0 grams of fat into kilocalories, and the kilocalories into joules
- Convert a sample containing 9.0 grams of carbohydrates into calories, then into kilocalories, then into joules
- Convert a 7.0 gram serving of protein into kilocalories, then into joules
- Convert a meal consisting of 14. grams of protein and 12. grams of carbohydrates into kilocalories, then joules
- Convert a full course serving of 8.0 grams of fat, 8.0 grams of protein and 6.0 grams of carbohydrates into kilocalories, then joules
(25) Study the following equations and determine whether they are endothermic or exothermic.
- \(C_6H_{12}O_{6\;(s)} + 6O_{2\; (g)} \rightarrow 6CO_{2\; (g)} + 6H_2O_{(l)} + \text{heat}\)
- \(H_2O_{(s)} + \text{heat} \rightarrow H_2O(l)\)
- \(C_{(s)} + 2I_{2\;(g)} \rightarrow CI_{4\;(s)} + \text{heat} \)
- \(NaOH_{(aq)} + HCl_{(aq)} \rightarrow NaCl_{(aq)} + H_2O_{(l)} +\text{heat} \)
- \(I_{2\;(s)} + \text{heat} \rightarrow I_{2\;(g)}\)
(26) Perform the following unit conversions.
- 17 joules to kilocalories
- 8.0 kilojoules to calories
- 900. calories to joules
- 200. kilocalories to kilojoules
- 5.5 kilojoules to kilocalories
(27) There are three primary dietary biomolecules that can be ingested to provide energy. Name each of them, and tell how many calories of energy each can provide. Which provides the most?
(28) For each of the following characteristics, determine whether they are more common of anabolic reactions or catabolic reactions.
- exothermic
- endothermic
- creating larger molecules out of more smaller ones
- breaking down large molecules into smaller ones
(29) Digestion of foods in the body releases energy. Is this a catabolic or anabolic reaction?
Kinetics: Reaction Rates
(30) Draw two reaction energy diagrams, one with the peak at a higher elevation than the other. Which one has the greater activation energy? Which one will proceed the fastest?
(31) Observe the given energy diagram and provide the requested information.
Energy 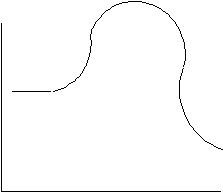
Reaction Progress
- Is this reaction exothermic or endothermic? How can you tell?
- Identify where the reactants lie on the diagram, and where the products lie.
- Draw a line to indicate the magnitude of the enthalpy (∆H) and indicate whether its value is positive or negative.
- Draw another line to indicate the activation energy (Ea).
- Label the transition state.
- Overlay a second curve using a dashed line to show how the reaction changes when a catalyst is added.
(32) Many things can affect the rate of a reaction. Determine what effect the following scenarios may have on a reaction.
- Addition of a catalyst
- Increasing the temperature
- Decreasing the concentration of the reactants
- Decreasing the temperature
- Increasing the concentration of the reactants
(33) Answer the following true or false statements.
- Catalysts need to be replaced periodically because they get used up in the reaction
- Catalysts change the reaction from exothermic to endothermic and vice-versa
- Catalysts change the size of the ∆H of a reaction
- A type of catalyst synthesized and utilized within the body is called an enzyme
- Catalysts are unaffected by the chemical reaction and do not participate in the balancing of the chemical equation
(34) An exothermic reaction occurs fairly slowly if unassisted by a catalyst. Draw two energy diagrams, one for the first reaction without its catalyst, and one more for the reaction after a catalyst has been added. What has changed between the two? Draw two more energy diagrams illustrating the same situation but with endothermic reactions instead. Is there any more significant difference between these other two diagrams?
Equilibrium & Le Chatlier’s Principle Questions
(35) Myoglobin is an oxygen storage protein found in muscle tissue. Diving mammals (dolphins, whales, seals, etc) contain 3 to 10 times the myoglobin found in humans. These increased levels of myglobin allow mammals to store additional oxygen in their muscle tissue. The additional oxygen reserves allow these diving mammals to stay under water for longer periods of times compared to humans who can only utilize the oxygen stored in the lungs. The reaction of myoglobin with oxygen is shown below.
\[Mb + O_2 \rightleftharpoons MbO_2\]
- What type of reaction is shown above – completion or equilibrium?
- Which direction does the reaction shift when the concentration of \(Mb\) is increased?
- Which direction does the reaction shift when the concentration of \(Mb\) is decreased?
- Which direction does the reaction shift when the concentration of \(O_2\) is increased?
- Which direction does the reaction shift when the concentration of \(O_2\) is decreased?
- Which direction does the reaction shift when the concentration of \(MbO_2\) is increased?
- Which direction does the reaction shift when the concentration of \(MbO_2\) is decreased?
(36) Methanol (CH3OH) can be formed from the equilibrium reaction of carbon monoxide with hydrogen gas.
- Write the balanced chemical reaction showing carbon monoxide and hydrogen as the reactants and methanol as the product. Both the reactants and products are in the gas phase. Pay attention to the reaction arrow.
- How is the equilibrium affected when the amount of carbon monoxide is increased?
- How is the equilibrium affected when the amount of hydrogen is decreased?
- How is the equilibrium affected when the pressure is increased?
- How is the equilibrium affected when the volume is increased?
Additional Exercises
(37) A half-mile walk will burn roughly 20. kcal. If you decide to start walking to and from school instead of driving four days a week and you live 1.5 miles away, how many additional kcal will you burn each week?
(38) An average person can maintain their weight on a 2.0x103 kcal/day diet. Some doctors recommend balancing the intake with a ratio of 35.% protein, 45.% carbohydrate and 20.% lipids. How many grams of each type of nutrient do these percentages represent?
(39) Some substances do not take on a liquid form between their solid and gaseous forms, such as carbon dioxide or iodine. The process of conversion from solid directly to gas is known as sublimation, and its reverse is known as deposition.
- Is sublimation more likely to be an exothermic or endothermic reaction?
- Is deposition more likely to be an exothermic or endothermic reaction?
- If you hold a bottle of iodine crystals in your bare hands and you see the crystals giving off some gas, would you expect your hands and/or the glass to feel hotter or colder? Explain.
(40) Propane gas is a popular form of fuel for barbecue grills. The molecular formula is C3H8. Write the chemical equation for this combustion reaction. Add in \text{heat} on whichever side is appropriate to indicate whether it is endothermic or exothermic.
(41) If the average male has a body mass of 70 kg, of which 60% is water, how many moles of water are in an average male?
(42) If the average female is 60.0 kg and contains 0.00174% iron, how many moles of iron are in an average female?
(43) How many moles of each ion are present in 2.67 mol of each compound?
- CaCl2
- Na2SO4
- Al(NO3)3
- Fe3(SO4)2
(44) How many moles of each element are present in 0.00445 mol of each compound?
- CH4
- CO2
- C3H8
- N2O5
-
If 6.63 × 10−6 mol of a compound has a mass of 2.151 mg, what is the molar mass of the compound?
-
Hemoglobin (molar mass is approximately 64,000 g/mol) is the major component of red blood cells that transports oxygen and carbon dioxide in the body. How many moles are in 0.034 g of hemoglobin?

