9.2: Endothermic and Exothermic Changes
- Page ID
- 52183
Skills to Develop
- Define potential energy and kinetic energy.
- Define endothermic and exothermic reactions.
- Describe how heat is transferred in endothermic and exothermic reactions.
- Determine whether a reaction is endothermic or exothermic through observations, temperature changes, or an energy diagram.
All Chemical Reactions Involve Energy
Remember that all chemical reactions involve a change in the bonds of the reactants. The bonds in the reactants are broken and the bonds of the products are formed. Chemical bonds have potential energy or "stored energy". Because we are changing the bonding, this means we are also changing how much of this "stored energy" there is in a reaction.
When chemical reactions occur, the new bonds formed never have exactly the same amount of potential energy as the bonds that were 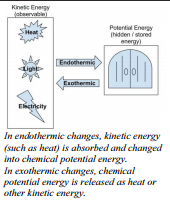 broken. Therefore, all chemical reactions involve energy changes. Energy is either given off by the reaction or energy is taken in by the reaction. There are many types of energy that can be involved in these changes. Different types of energy include:
broken. Therefore, all chemical reactions involve energy changes. Energy is either given off by the reaction or energy is taken in by the reaction. There are many types of energy that can be involved in these changes. Different types of energy include:
- Heat
- Electricity
- Light
- Chemical potential energy
Sometimes the products have more energy stored in their bonds than the reactants had to start with. This means that the reaction started with less hidden energy than we had at the end. Where did this extra energy come from? In these reactions, heat or other forms of energy are absorbed by the reactants from the surroundings to supply some of this hidden bond energy. These reactions are called endothermic reactions. Endothermic reactions absorb heat or other forms of energy from their surroundings.
Sometimes the products have less energy stored in their bonds than the reactants had to start with. This means that the reaction started with more hidden energy than we had at the end. Where did this extra energy go? In these reactions, heat or other forms of energy are released by the reactants from the surroundings to give off some of this extra hidden bond energy. These reactions are called exothermic reactions. Exothermic reactions release heat or other forms of energy into their surroundings.
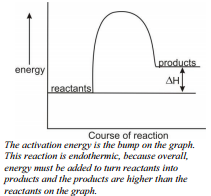 The last time you went camping, you might have lit a campfire. The burning of the wood in the fire pit released energy into the environment. But like lighting the fire at the camp, chemical reactions need a minimum amount of energy in order for a reaction to begin. Your campfire won't start until you supply a bit of energy, the initial match, to get it started. Before any reaction can occur, reactant bonds need to be broken. A minimum amount of energy, the activation energy, must be supplied before any reaction can take place. This is different than labeling a reaction as endothermic or exothermic. Although sometimes it is necessary to give a little energy to a reaction to start, if more energy is given off than you put in to get it started, the reaction is overall exothermic. If you give the reaction more energy than it gives you, the reaction is endothermic.
The last time you went camping, you might have lit a campfire. The burning of the wood in the fire pit released energy into the environment. But like lighting the fire at the camp, chemical reactions need a minimum amount of energy in order for a reaction to begin. Your campfire won't start until you supply a bit of energy, the initial match, to get it started. Before any reaction can occur, reactant bonds need to be broken. A minimum amount of energy, the activation energy, must be supplied before any reaction can take place. This is different than labeling a reaction as endothermic or exothermic. Although sometimes it is necessary to give a little energy to a reaction to start, if more energy is given off than you put in to get it started, the reaction is overall exothermic. If you give the reaction more energy than it gives you, the reaction is endothermic.
Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation 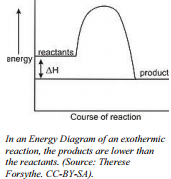 energy. If, on an energy diagram, the products have more stored energy than the reactants started with, the reaction is endothermic.. You had to give the reaction energy. If, on the energy diagram, the products have less stored energy than the reactants started with, the reaction is exothermic.
energy. If, on an energy diagram, the products have more stored energy than the reactants started with, the reaction is endothermic.. You had to give the reaction energy. If, on the energy diagram, the products have less stored energy than the reactants started with, the reaction is exothermic.
Another way of classifying a reaction as endothermic or exothermic was already presented to you in Chapter 7. Remember what was said about \(\Delta H\)? If \(\Delta H\) had a positive value, the reaction is endothermic. This means that energy must be added to the reactants in order for the reaction to occur. If \(\Delta H\) has a negative value, the reaction is exothermic and energy is produced with the rest of the products and given off into the surroundings.
Consider the following equation:
\[2 \ce{HgO} \rightarrow 2 \ce{Hg} + \ce{O_2} \: \: \: \: \: \Delta H = +181.7 \: \text{kJ}\]
The positive sign \(\left( + \right)\) on the \(\Delta H\) tells us that the reaction is endothermic, that more energy had to be added to the reaction and that there is less energy stored in the bonds of the reactant (mercury (II) oxide) than is stored in the bonds of the products. Therefore, extra energy had to be added to the reaction to form the products.
Contrast the previous reaction to the next reaction:
\[\ce{NaCl} + \ce{AgNO_3} \rightarrow \ce{AgCl} + \ce{NaNO_3} \: \: \: \: \: \Delta H = -166 \: \text{kJ}\]
This is an example of a chemical reaction in which energy is released. This means that there is less energy stored in the bonds of the products than there was in the bonds in the reactants. Therefore, extra energy was left over when the reactants become the products. The negative sign \(\left( - \right)\) on the \(\Delta H\) tells us that this reaction had extra energy. This reaction is exothermic, meaning that energy is released. Another way to think of this is that energy is a product, it is something produced, or released, in the reaction.
Example 9.2.1
Label each of the following processes as endothermic or exothermic.
a) water boiling
b) gasoline burning
c) ice forming on a pond
d) \(2 \ce{SO_2} + \ce{O_2} \rightarrow 2 \ce{SO_3} \: \: \: \: \: \Delta H = -46.8 \: \text{kJ/mol}\)
Solution:
a) endothermic - you must put a pan of water on the stove and give it heat in order to get water to boil. Because you are adding heat/energy, the reaction is endothermic.
b) exothermic - when you burn something, it feels hot to you because it is giving off heat into the surroundings.
c) exothermic - think of ice forming in your freezer instead. You put water into the freezer, which takes heat out of the water, to get it to freeze. Because heat is being pulled out of the water, it is exothermic. Heat is leaving.
d) This reaction is exothermic, because \(\Delta H\) is negative.
Lesson Summary
- All chemical reactions involve changes in energy. This may be a change in heat, electricity, light, or other forms of energy.
- Reactions that absorb energy are endothermic.
- Reactions that release energy are exothermic.
Vocabulary
- Potential energy: The energy of position or stored energy, including bond energy.
- Endothermic: Reactions in which energy is absorbed.
- Exothermic: Reactions in which energy is released.

