7.6: Stoichiometry
- Page ID
- 51270
Skills to Develop
- Explain the meaning of the term "stoichiometry".
- Determine the mole ratios in chemical equations.
- Calculate the number of moles of any reactant or product from a balanced equation given the number of moles of one reactant or product.
- Calculate the mass of any reactant or product from a balanced equation given the mass of one reactant or product.
You have learned that chemical equations provide us with information about the types of particles that react to form products. Chemical equations also provide us with the relative number of particles and moles that react to form products. In this section you will explore the quantitative relationships that exist between the quantities of reactants and products in a balanced equation. This is known as stoichiometry.
Stoichiometry, by definition, is the calculation of the quantities of reactants or products in a chemical reaction using the relationships found in the balanced chemical equation. The word stoichiometry is actually Greek from two words: \(\sigma \tau \omicron \iota \kappa \eta \iota \omicron \nu\), which means "element", and \(\mu \epsilon \tau \rho \omicron \nu), which means "measure".
Interpreting Chemical Equations
The mole, as you remember, is a quantitative measure that is equivalent to Avogadro's number of particles. So how does this relate to the chemical equation? Look at the chemical equation below.

The coefficients used, as we have learned, tell us the relative amounts of each substance in the equation. So for every 2 units of copper (II) sulfate (\(\ce{CuSO_4}\)) we have, we need to have 4 units of potassium iodide (\(\ce{KI}\)). For every two dozen copper (II) sulfates, we need 4 dozen potassium iodides. Because the unit "mole" is also a counting unit, we can interpret this equation in terms of moles, as well: For every two moles of copper (II) sulfate, we need 4 moles potassium iodide.
Look at the chemical equation below. This reaction can be interpreted many ways.
\[\ce{N_2O_3} + \ce{H_2O} \rightarrow 2 \ce{HNO_2}\]
- One molecule of dinitrogen trioxide plus one molecule of water yields two molecules of hydrogen nitrite.
- One mole of dinitrogen trioxide plus one mole of water yields two moles of hydrogen nitrite.
Example \(\PageIndex{1}\)
For each of the following equations, indicate the number of formula units or molecules, and the number of moles present in the balanced equation.
- \(2 \ce{C_2H_6} + 7 \ce{O_2} \rightarrow 4 \ce{CO_2} + 6 \ce{H_2O}\)
- \(\ce{KBrO_3} + 6 \ce{KI} + 6 \ce{HBr} \rightarrow 7 \ce{KBr} + 3 \ce{H_2O}\)
Solution:
- Two molecules of \(\ce{C_2H_6}\) plus seven molecules of \(\ce{O_2}\) yields four molecules of \(\ce{CO_2}\) plus six molecules of \(\ce{H_2O}\). Two moles of \(\ce{C_2H_6}\) plus seven moles of \(\ce{O_2}\) yields four moles of \(\ce{CO_2}\) plus six moles of \(\ce{H_2O}\).
- One molecules of \(\ce{KBrO_3}\) plus six molecules of \(\ce{KI}\) plus six molecules of \(\ce{HBr}\) yields seven molecules of \(\ce{KBr}\) plus three molecules of \(\ce{I_2}\) and three molecules of \(\ce{H_2O}\). One mole of \(\ce{KBrO_3}\) plus six moles of \(\ce{KI}\) plus six moles of \(\ce{HBr}\) yields seven moles of \(\ce{KBr}\) plus three moles of \(\ce{I_2}\) plus three moles of \(\ce{H_2O}\).
In chemistry, we "talk" to each other using chemical equations, the same way mathematicians talk to each other using mathematical equations. In chemistry, we also want to talk about quantities. Using stoichiometry, you can predict the quantities of reactants as products that can be used and produced in a chemical reaction. This requires working with balanced chemical equations.
In a previous section we explored mole relationships in balanced chemical equations. In this section, we will use the mole as a conversion factor to calculate moles of product from a given number of moles of reactant or moles of reactant from a given number of moles of product. This is called a "mole-mole" calculation. We will also perform "mass-mass" calculations, which allow you to determine the mass of reactant you require to produce a given amount of product or to calculate the mass of product you can obtain from a given mass of reactant.
Mole Ratios
A mole ratio is the relationship of the number of moles of the substances in a reaction. For instance, in the following reaction we read the coefficients as molecules (or formula units) and moles:
\[2 \ce{H_2} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{H_2O} \left( l \right)\]
2 moles of \(\ce{H_2}\) react with 1 mole of \(\ce{O_2}\) to produce 2 moles of \(\ce{H_2O}\). Or, an alternate method to represent this information is with mole ratios. The following mole ratios can be obtained from this reaction:
\[\frac{2 \: \text{mol} \: \ce{H_2}}{1 \: \text{mol} \: \ce{O_2}} \: \text{or} \: \frac{1 \: \text{mol} \: \ce{O_2}}{2 \: \text{mol} \: \ce{H_2}} \: \text{or} \: \frac{2 \: \text{mol} \: \ce{H_2O}}{2 \: \text{mol} \: \ce{H_2}} \: \text{or} \: \frac{2 \: \text{mol} \: \ce{H_2O}}{1 \: \text{mol} \: \ce{O_2}} \: \text{or} \: \frac{2 \: \text{mol} \: \ce{H_2}}{2 \: \text{mol} \: \ce{H_2O}} \: \text{or} \: \frac{1 \: \text{mol} \: \ce{O_2}}{2 \: \text{mol} \: \ce{H_2O}}\]
Using the coefficients of a balanced reaction, you can compare any two substances in the reaction you are interested in, whether they are reactants or products. The correct mole ratios of the reactants and products in a chemical equation are determined by the balanced equation. Therefore, the chemical equation MUST always be balanced before the mole ratios are used for calculations.
Mole-Mole Calculations
We have already learned the process through which chemists solve many math problems, the factor-label method. The mole-mole ratio we obtain from a balanced reaction can be used as a ratio in part of that process.
Example \(\PageIndex{2}\)
If only \(0.050 \: \text{mol}\) of magnesium hydroxide, \(\ce{Mg(OH)_2}\), is present, how many moles of phosphoric acid, \(\ce{H_3PO_4}\), would be required for the reaction?
\[2 \ce{H_3PO_4} + 3 \ce{Mg(OH)_2} \rightarrow \ce{Mg_3(PO_4)_2} + 6 \ce{H_2O} \nonumber\]
Solution:
We need to set up this problem using the same steps of dimensional analysis.
Given: \(0.050 \: \text{mol} \: \ce{Mg(OH)_2}\)
Find: \(\text{mol} \: \ce{H_3PO_4}\)
The ratio we need is one that compares \(\text{mol} \: \ce{Mg(OH)_2}\) to \(\text{mol} \: \ce{H_3PO_4}\). This is the ratio obtained in the balanced reaction. Note that there are other reactants and products in this reaction, but we don't need to use them to solve this problem.
\[0.050 \: \cancel{\text{mol} \: \ce{Mg(OH)_2}} \cdot \frac{2 \: \text{mol} \: \ce{H_3PO_4}}{3 \: \cancel{\text{mol} \: \ce{Mg(OH)_2}}} = 0.033 \: \text{mol} \: \ce{H_3PO_4} \nonumber\]
Notice if the equation was not balanced, the amount of \(\ce{H_3PO_4}\) would have been different. The reaction MUST be balanced to use the reaction in any calculations. As you can see, the mole ratios are useful for converting between the number of moles of one substance and another.
Calculations Using a Mole Map
Being able to perform mass-mass calculations allows you to determine the mass of reactant (how many grams) you require to produce a given amount of product, or to calculate the mass of product you can obtain from a given mass of reactant or the mass of reactant needed to react with a specific amount of another reactant. Just as when working with mole ratios, it is important to make sure you have a balanced chemical equation before you begin.
These types of problems can be done using dimensional analysis, also called the factor-label method. This is simply a method that uses conversion factors to convert from one unit to another. In this method, we can follow the cancelation of units to the correct answer.
For example, \(15.0 \: \text{g}\) of chlorine gas is bubbled over liquid sulfur to produce disulfur dichloride. How much sulfur, in grams, is needed according to the balanced equation:
\[\ce{Cl_2} \left( g \right) + 2 \ce{S} \left( l \right) \rightarrow \ce{S_2Cl_2} \]
- Identify the given: \(15.0 \: \text{g} \: \ce{Cl_2}\)
- Identify the find: \(\text{g} \: \ce{S}\)
- Next, use the correct ratios that allow you to cancel the units you don't want and get to the unit you are calculating for.
\[15.0 \: \cancel{\text{g} \: \ce{Cl_2}} \times \frac{1 \: \cancel{\text{mol} \: \ce{Cl_2}}}{71.0 \: \cancel{\text{g} \: \ce{Cl_2}}} \times \frac{2 \: \cancel{\text{mol} \: \ce{S}}}{1 \: \cancel{\text{mol} \: \ce{Cl_2}}} \times \frac{32.1 \: \text{g} \: \ce{S}}{1 \: \cancel{\text{mol} \: \ce{S}}} = 13.6 \: \text{g} \: \ce{S}\]
If we combine the mole-mole ratio with ratios we learned previously, when we first learned about the mole, we have several ratios we can use to solve a wide variety of problems. The mole map is a tool we can use to help us to know which ratios to use when solving problems.
You use this map much like you would use a road map. You must first find out where you are on the map (your given units) and where you would like to go (your "find" units). The map will then let you know which roads (ratios) to take to get there. Let's see how this works with a couple of example problems.
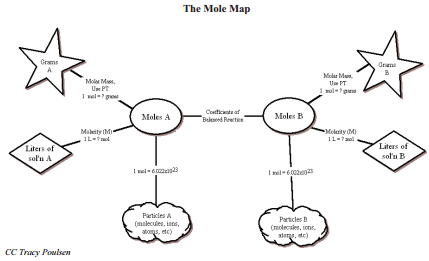
Example \(\PageIndex{3}\)
The thermite reaction is a very exothermic reaction which produces liquid iron, given by the following balanced equation:
\[\ce{Fe_2O_3} \left( s \right) + 2 \ce{Al} \left( s \right) \rightarrow 2 \ce{Fe} \left( l \right) + \ce{Al_2O_3} \left( s \right) \nonumber\]
If \(5.00 \: \text{g}\) of iron is produced, how much iron (III) oxide was placed in the original container?
Solution:
1) Identify the "given": \(5.00 \: \text{g}\) iron. (Even though this is a product, it is still the measurement given to us in the problem.)
 2) Identify the units of the "find": \(\text{g} \: \ce{Fe_2O_3}\) (Remember, mass is measured in grams.)
2) Identify the units of the "find": \(\text{g} \: \ce{Fe_2O_3}\) (Remember, mass is measured in grams.)
3) Ratios: This is where the map comes in handy. To start with, we are at \(5.00 \: \text{g} \: \ce{Fe}\). For this problem, then, "A" on the map stands for \(\ce{Fe}\). We start at grams A.
We want to know \(\text{g} \: \ce{Fe_2O_3}\). For this problem, "B" stands for \(\ce{Fe_2O_3}\). We are heading to grams B.
Our map tells us this problem will take 3 ratios (3 roads from \(\text{g}\) A to \(\text{g}\) B): molar mass of A, mole-mole ratio from a balanced reaction, and molar mass of B. To solve our problem, the work will look like:
\[5.00 \: \cancel{\text{g} \: \ce{Fe}} \cdot \frac{1 \: \cancel{\text{mol} \: \ce{Fe}}}{55.85 \: \cancel{\text{g} \: \ce{Fe}}} \cdot \frac{1 \: \cancel{\text{mol} \: \ce{Fe_2O_3}}}{2 \: \cancel{\text{mol} \: \ce{Fe}}} \cdot \frac{159.7 \: \text{g} \: \ce{Fe_2O_3}}{1 \: \cancel{\text{mol} \: \ce{Fe_2O_3}}} = 7.17 \: \text{g} \: \ce{Fe_2O_3}\]
Example 7 \(\PageIndex{4}\)
Ibuprofen is a common painkiller used by many people around the globe. It has the formula \(\ce{C_{13}H_{18}O_2}\). If \(200.0 \: \text{g}\) of Ibuprofen is combusted how much carbon dioxide is produced? The balanced reaction is:
\[2 \ce{C_{13}H_{18}O_2} + 33 \: \ce{O_2} \rightarrow 26 \ce{CO_2} + 18 \ce{H_2O} \nonumber\]
Solution:
Given: \(200.0 \: \text{g} \: \ce{C_{13}H_{18}O_2}\) (\(\text{g}\) A on map)
Find: \(\text{g} \: \ce{CO_2}\) (\(\text{g}\) B on map)
Ratios: The map says we need to use the molar mass of \(\ce{C_{13}H_{18}O_2}\), then the coefficients of the balanced reaction, then the molar mass of \(\ce{CO_2}\).
\[200.0 \: \cancel{\text{g} \: \ce{C_{13}H_{18}O_2}} \cdot \frac{1 \: \cancel{\text{mol} \: \ce{C_{13}H_{18}O_2}}}{206.3 \: \cancel{\text{g} \: \ce{C{13}H_{18}O_2}}} \cdot \frac{26 \: \cancel{\text{mol} \: \ce{CO_2}}}{2 \: \cancel{\text{mol} \: \ce{C_{13}H_{18}O_2}}} \cdot \frac{44.01 \: \text{g} \: \ce{CO_2}}{1 \: \cancel{\text{mol} \: \ce{CO_2}}} = 555 \: \text{g} \: \ce{CO_2}\]
Example \(\PageIndex{5}\)
If sulfuric acid is mixed with sodium cyanide, the deadly gas hydrogen cyanide is produced. How many moles of sulfuric acid would have been placed in the container to produce \(12.5 \: \text{g}\) of hydrogen cyanide? The balanced reaction is:
\[2 \ce{NaCN} + \ce{H_2SO_4} \rightarrow \ce{Na_2SO_4} + 2 \ce{HCN}\]
Solution:
Given: \(12.5 \: \text{g} \: \ce{HCN}\) (\(\text{g}\) A on map)
Find: \(\text{mol} \: \ce{H_2SO_4}\) (\(\text{mol}\) B on map)
Ratios: The mole map says we need the molar mass of \(\ce{HCN}\) and the coefficients of the balanced reaction.
\[12.5 \: \cancel{\text{g} \: \ce{HCN}} \cdot \frac{1 \: \cancel{\text{mol} \: \ce{HCN}}}{27.0 \: \cancel{\text{g} \: \ce{HCN}}} \cdot \frac{1 \: \text{mol} \: \ce{H_2SO_4}}{2 \: \cancel{\text{mol} \: \ce{HCN}}} = 0.231 \: \text{mol} \: \ce{H_2SO_4}\]
Example \(\PageIndex{6}\)
How many atoms of carbon would be released from the complete dehydration of \(18.0 \: \text{g}\) of sugar (\(\ce{C_5H_{12}O_6}\)) with sulfuric acid? The balanced reaction is:
\[\ce{C_6H_{12}O_6} + \ce{H_2SO_4} \rightarrow 6 \ce{C} + 7 \ce{H_2O} + \ce{SO_3}\]
Solution:
Given: \(18 \: \text{g} \: \ce{C_6H_{12}O_6}\)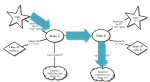
Find: atoms \(\ce{C}\)
Ratios: The mole map says we need the molar mass of the sugar, the balanced reaction, and finally Avogadro's number.
\[18.0 \: \cancel{\text{g} \: \ce{C_6H_{12}O_6}} \cdot \frac{1 \: \cancel{\text{mol} \: \ce{C_6H_{12}O_6}}}{180 \: \cancel{\text{g} \: \ce{C_6H_{12}O_6}}} \cdot \frac{6 \: \cancel{\text{mol} \: \ce{C}}}{1 \: \cancel{\text{mol} \: \ce{C_6H_{12}O_6}}} \cdot \frac{6.02 \times 10^{23} \: \text{atoms} \: \ce{C}}{1 \: \cancel{\text{mol} \: \ce{C}}} = 3.6 \times 10^{23} \: \text{atoms} \: \ce{C}\]
Summary
Stoichiometry is the calculation of the quantities of reactants or products in a chemical reaction using the relationships found in the balanced chemical equation. The coefficients in a balanced chemical equation represent the reacting ratios of the substances in the reaction. The coefficients of the balanced equation can be used to determine the ratio of moles of all substances in a reaction.
Vocabulary
- Stoichiometry: The calculation of quantitative relationships of the reactants and products in a balanced chemical equation.
- Formula unit: The empirical formula of an ionic compound.
- Mole ratio: The ratio of the moles of one reactant or product to the moles of another reactant or product according to the coefficients in the balanced chemical equation.
Contributors

