7.5: Types of Reactions
- Page ID
- 50645
Skills to Develop
- Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction.
- Predict the products of simple reactions.
Chemical reactions are classified into types to help us analyze them and also to help us predict what the products of the reaction will be. The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion.
Synthesis Reactions
A synthesis reaction is one in which two or more reactants combine to make one type of product.
General equation: \(\text{A} + \text{B} \rightarrow \text{AB}\)
Synthesis reactions occur as a result of two or more simpler elements or molecules combining to form a more complex molecule. Look at the example below. Here two elements (hydrogen and oxygen) are combining to for one product (water).
Example: \(2 \ce{H_2} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{H_2O} \left( l \right)\)
We can always identify a synthesis reaction because there is only one product of the reaction.
You should be able to write the chemical equation for a synthesis reaction if you are given a product by picking out its elements and writing the equation. Also, if you are given elemental reactants and told that the reaction is a synthesis reaction, you should be able to predict the products.
Example \(\PageIndex{1}\)
a) Write the chemical equation for the synthesis of silver bromide, \(\ce{AgBr}\).
b) Predict the products for the following reaction: \(\ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right)\)
Solution:
a) \(2 \ce{Ag} + \ce{Br_2} \rightarrow 2 \ce{AgBr}\)
b) \(\ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right) \rightarrow \ce{H_2CO_3}\)
Decomposition Reactions
When one type of reactant breaks down to form two or more products, we have a decomposition reaction. The best way to remember a decomposition reaction is that for all reactions of this type, there is only one reactant.
General equation: \(\text{AB} \rightarrow \text{A} + \text{B}\)
 Look at the example below for the decomposition of ammonium nitrate to dinitrogen oxide and water.
Look at the example below for the decomposition of ammonium nitrate to dinitrogen oxide and water.
Example: \(\ce{NH_4NO_3} \rightarrow \ce{N_2O} + 2 \ce{H_2O}\)
Notice the one reactant, \(\ce{NH_4NO_3}\), is on the left of the arrow and there is more than one on the right side of the arrow. This is the exact opposite of the synthesis reaction type.
When studying decomposition reactions, we can predict reactants in a similar manner as we did for synthesis reactions. Look at the formula for magnesium nitride, \(\ce{Mg_3N_2}\). What elements do you see in this formula? You see magnesium and nitrogen. Now we can write a decomposition reaction for magnesium nitride. Notice there is only one reactant.
\[\ce{Mg_3N_2} \rightarrow 3 \ce{Mg} + \ce{N_2}\]
Example \(\PageIndex{2}\)
Write the chemical equation for the decomposition of:
- \(\ce{Al_2O_3}\)
- \(\ce{Ag_2S}\)
- \(\ce{MgO}\)
Solution
- \(2 \ce{Al_2O_3} \rightarrow 4 \ce{Al} + 3 \ce{O_2}\)
- \(\ce{Ag_2S} \rightarrow 2 \ce{Ag} + \ce{S}\)
- \(2 \ce{MgO} \rightarrow 2 \ce{Mg} + \ce{O_2}\)
Single Replacement Reactions
 A third type of reaction is the single replacement reaction. In single replacement reactions one element reacts with one compound to form products. The single element is said to replace an element in the compound when products form, hence the name single replacement. Metal elements will always replace other metals in ionic compounds or hydrogen in an acid. Nonmetal elements will always replace another nonmetal in an ionic compound.
A third type of reaction is the single replacement reaction. In single replacement reactions one element reacts with one compound to form products. The single element is said to replace an element in the compound when products form, hence the name single replacement. Metal elements will always replace other metals in ionic compounds or hydrogen in an acid. Nonmetal elements will always replace another nonmetal in an ionic compound.
General equation: \(\text{A} + \text{BC} \rightarrow \text{B} + \text{AC}\)
Consider the following examples.
\[\ce{Zn} \left( s \right) + \ce{Cu(NO_3)_2} \left( aq \right) \rightarrow \ce{Zn(NO_3)_2} \left( aq \right) + \ce{Cu} \left( s \right)\]
Notice that the metal element, \(\ce{Zn}\), replaced the metal in the compound \(\ce{Cu(NO_3)_2}\). A metal element will always replace a metal in an ionic compound. Also, note that the charges of the ionic compounds must equal zero. To correctly predict the formula of the ionic product, you must know the charges of the ions you are combining.
\[\ce{Zn} \left( s \right) + 2 \ce{HBr} \left( aq \right) \rightarrow \ce{ZnBr_2} \left( aq \right) + \ce{H_2} \left( g \right)\]
When a metal element is mixed with acid, the metal will replace the hydrogen in the acid and release hydrogen gas as a product. Once again, note that the charges of the ionic compounds must equal zero. To correctly predict the formula of the ionic product, you must know the charges of the ions you are combining, in this case \(\ce{Zn^{2+}}\) and \(\ce{Br^-}\).
\[\ce{Cl_2} \left( g \right) + 2 \ce{KI} \left( aq \right) \rightarrow 2 \ce{KCl} \left( aq \right) + \ce{I_2} \left( s \right)\]
When a nonmetal element is added to an ionic compound, the element will replace the nonmetal in the compound. Also, to correctly write the formulas of the products, you must first identify the charges of the ions that will be in the ionic compound.
Example \(\PageIndex{3}\)
What would be the products of the reaction between solid aluminum and iron (III) oxide? The reactants are: \(\ce{Al} + \ce{Fe_2O_3} \rightarrow\)
Solution: In order to predict the products we need to know that aluminum will replace iron and form aluminum oxide (the metal will replace the metal ion in the compound). Aluminum has a charge of \(+3\) and oxygen has a charge of \(-2\). The compound formed between aluminum and oxygen, therefore, will be \(\ce{Al_2O_3}\). Since iron is replaced in the compound by aluminum, the iron will now be the single element in the products. The unbalance equation will be:
\[\ce{Al} + \ce{Fe_2O_3} \rightarrow \ce{Al_2O_3} + \ce{Fe}\]
and the balanced equation will be:
\[2 \ce{Al} + \ce{Fe_2O_3} \rightarrow \ce{Al_2O_3} + 2 \ce{Fe} \nonumber\]
Example \(\PageIndex{4}\)
- Write the chemical equation for the single replacement reaction between zinc solid and lead (II) nitrate solution to produce zinc nitrate solution and solid lead. (*Note: zinc forms ions with a \(+2\) charge)
- Predict the products for the following reaction: \(\ce{Fe} + \ce{CuSO_4}\) (in this reaction, assume iron forms ions with a \(+2\) charge)
- Predict the products for the following reaction: \(\ce{Al} + \ce{CuCl_2}\)
- Complete the following reaction. Then balance the equation: \(\ce{Al} + \ce{HNO_3} \rightarrow\)
Solution:
- \(\ce{Zn} + \ce{Pb(NO_3)_2} \rightarrow \ce{Pb} + \ce{Zn(NO_3)_2}\)
- \(\ce{Fe} + \ce{CuSO_4} \rightarrow \ce{Cu} + \ce{FeSO_4}\)
- \(2 \ce{Al} + 3 \ce{CuCl_2} \rightarrow 3 \ce{Cu} + 2 \ce{AlCl_3}\)
- \(2 \ce{Al} + 6 \ce{HNO_3} \rightarrow 2 \ce{Al(NO_3)_3} + 3 \ce{H_2}\)
Double Replacement Reactions
For double replacement reactions two ionic compound reactants will react by having the cations exchange places, forming two new ionic compounds. The key to this type of reaction, as far as identifying it over the other types, is that is has two compounds as reactants. This type of reaction is more common than any of the others and there are many different types of double replacement reactions. Precipitation and neutralization reactions are two of the most common double replacement reactions. Precipitation reactions are ones where two aqueous compound reactants combine to form products where one of the products is an insoluble solid. A neutralization reaction is one where the two reactant compounds are an acid and a base and the two products are a salt and water (i.e. \(\text{acid} + \text{base} \rightarrow \text{salt} + \text{water}\)).
reactants. This type of reaction is more common than any of the others and there are many different types of double replacement reactions. Precipitation and neutralization reactions are two of the most common double replacement reactions. Precipitation reactions are ones where two aqueous compound reactants combine to form products where one of the products is an insoluble solid. A neutralization reaction is one where the two reactant compounds are an acid and a base and the two products are a salt and water (i.e. \(\text{acid} + \text{base} \rightarrow \text{salt} + \text{water}\)).
General equation: \(\text{AB} + \text{CD} \rightarrow \text{AD} + \text{BC}\)
For example, when solutions of silver nitrate and sodium chloride are mixed, the following reaction occurs:
\[\ce{AgNO_3} \left( aq \right) + \ce{NaCl} \left( aq \right) \rightarrow \ce{AgCl} \left( s \right) + \ce{NaNO_3} \left( aq \right)\]
This is an example of a precipitate reaction. Notice that two aqueous reactants form one solid, the precipitate, and another aqueous product.
An example of a neutralization reaction occurs when sodium hydroxide, a base, is mixed with sulfuric acid:
\[2 \ce{NaOH} \left( aq \right) + \ce{H_2SO_4} \left( aq \right) \rightarrow \ce{Na_2SO_4} \left( aq \right) + 2 \ce{H_2O} \left( l \right)\]
In order to write the products for a double replacement reaction, you must be able to determine the correct formulas for the new compounds. Remember, the total charge of all ionic compounds is zero. To correctly write the formulas of the products, you must know the charges of the ions in the reactants. Let's practice with an example or two.
Example \(\PageIndex{5}\)
A common laboratory experiment involves the reaction between lead (II) nitrate and sodium iodide, both colorless solutions. The reactants are given below. Predict the products.
\[\ce{Pb(NO_3)_2} \left( aq \right) + \ce{NaI} \left( aq \right) \rightarrow \nonumber\]
Solution
We know that the cations exchange anions. We now have to look at the charges of each of the cations and anions to see what the products will be. In \(\ce{Pb(NO_3)_2}\), the nitrate, \(\ce{NO_3^-}\), has a charge of \(-1\). This means the lead must be \(+2\), \(\ce{Pb^{2+}}\). In the sodium iodide, we are combining \(\ce{Na^+}\) and \(\ce{I^-}\).
Now we switch ions and write the correct subscripts so the total charge of each compound is zero. The \(\ce{Pb^{2+}}\) will combine with the \(\ce{I^-}\) to form \(\ce{PbI_2}\). The \(\ce{Na^+}\) will combine with the \(\ce{NO_3^-}\) to form \(\ce{NaNO_3}\).
Only after we have the correct formulas can we worry about balancing the two sides of the reaction. The final balanced reaction will be:
\[\ce{Pb(NO_3)_2} \left( aq \right) + 2 \ce{NaI} \left( aq \right) \rightarrow \ce{PbI_2} \left( s \right) + 2 \ce{NaNO_3} \left( aq \right)\]
Example \(\PageIndex{6}\)
- Write a chemical reaction for the double replacement reaction between calcium chloride solution and potassium hydroxide solution to produce potassium chloride solution and a precipitate of calcium hydroxide.
- Predict the products for the following reaction: \(\ce{AgNO_3} \left( aq \right) + \ce{NaCl} \left( aq \right) \rightarrow\)
- Predict the products for the following reaction: \(\ce{FeCl_3} \left( aq \right) + \ce{KOH} \left( aq \right) \rightarrow\)
Solution:
- \(\ce{CaCl_2} \left( aq \right) + 2 \ce{KOH} \left( aq \right) \rightarrow \ce{Ca(OH)_2} \left( s \right) + 2 \ce{KCl} \left( aq \right)\)
- \(\ce{AgNO_3} \left( aq \right) + \ce{NaCl} \left( aq \right) \rightarrow \ce{AgCl} \left( s \right) + \ce{NaNO_3} \left( aq \right)\)
- \(\ce{FeCl_3} \left( aq \right) + \ce{KOH} \left( aq \right) \rightarrow \ce{Fe(OH)_3} \left( s \right) + \ce{KCl} \left( aq \right)\)
Combustion
I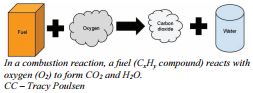 n a combustion reaction oxygen reacts with another substance to produce carbon dioxide and water. This is what happens when fuel burns. In a particular branch of chemistry, known as organic chemistry, we study compounds known as hydrocarbons. A hydrocarbon is a compound consisting of only hydrogen and carbon. Hydrocarbons represent the major components of all organic material including fuels. Combustion reactions usually have the same products, \(\ce{CO_2}\) and \(\ce{H_2O}\), and one of its reactants is always oxygen. In other words, the only part that changes from one combustion reaction to the next is the actual hydrocarbon that burns. The general equation is given below. Notice the oxygen, carbon dioxide, and water parts of the reaction are listed for you to show you how these reactants and products remain the same from combustion reaction to combustion reaction.
n a combustion reaction oxygen reacts with another substance to produce carbon dioxide and water. This is what happens when fuel burns. In a particular branch of chemistry, known as organic chemistry, we study compounds known as hydrocarbons. A hydrocarbon is a compound consisting of only hydrogen and carbon. Hydrocarbons represent the major components of all organic material including fuels. Combustion reactions usually have the same products, \(\ce{CO_2}\) and \(\ce{H_2O}\), and one of its reactants is always oxygen. In other words, the only part that changes from one combustion reaction to the next is the actual hydrocarbon that burns. The general equation is given below. Notice the oxygen, carbon dioxide, and water parts of the reaction are listed for you to show you how these reactants and products remain the same from combustion reaction to combustion reaction.
General equation: \(\ce{C}_x \ce{H}_y \: \text{(hydrocarbon)} \: + \ce{O_2} \rightarrow \ce{CO_2} + \ce{H_2O}\)
Look at the reaction for the combustion of octane, \(\ce{C_8H_{18}}\), below. Octane has 8 carbon atoms hence the prefix "oct".
Example: \(2 \ce{C_8H_{18}} + 25 \ce{O_2} \rightarrow 16 \ce{CO_2} + 18 \ce{H_2O}\)
This reaction is referred to as complete combustion. Complete combustion reactions occur when there is enough oxygen to burn the entire hydrocarbon. This is why there are only carbon dioxide and water as products.
Example \(\PageIndex{7}\)
Write the balanced reaction for the complete combustion of propane, \(\ce{C_3H_8}\).
Solution:
The reactants of all combustion reactions include the fuel (a compound with carbon and hydrogen) reacting with oxygen. The products are always carbon dioxide and water.
\[\ce{C_3H_8} + 5 \ce{O_2} \rightarrow 3 \ce{CO_2} + 4 \ce{H_2O} \nonumber\]
Lesson Summary
| The Five Types of Chemical Reactions | |
| Reaction Name | Reaction Description |
| Synthesis |
Two or more reactants form one product. |
| Decomposition | One type of reactant forms two or more products. |
| Single Replacement | One element reacts with one compound to form products |
| Double Replacement | Two compounds act as reactants. |
| Combustion | A fuel reactant reacts with oxygen. |
Vocabulary
- Synthesis reaction: A reaction in which two or more reactants combine to make one product.
- Decomposition reaction: A reaction in which one reactant breaks down to form two or more products.
- Single replacement reaction: A reaction in which an element reacts with a compound to form products.
- Double replacement reaction: A reaction in which two reactants form products by having the cations exchange places with the anions.
- Combustion reaction: A reaction in which oxygen reacts with another substance to produce carbon dioxide and water.
- Hydrocarbon: An organic substance consisting of only hydrogen and carbon.

