7.1: Chemical and Physical Change
- Page ID
- 50542
Skills to Develop
- Label a change as chemical or physical.
- List evidence that can indicate a chemical change occurred.
Change is happening all around us all of the time. Just as chemists have classified elements and compounds, they have also classified types of changes. Changes are either classified as physical or chemical changes.
Physical & Chemical Change
Chemists learn a lot about the nature of matter by studying the changes that matter can undergo. Chemists make a distinction between two different types of changes that they study - physical changes and chemical changes. Physical changes are changes in which no bonds are broken 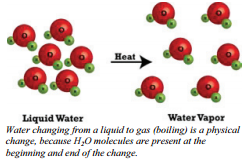 or formed. This means that the same types of compounds or elements that were there at the beginning of the change are there at the end of the change. Because the ending materials are the same as the beginning materials, the properties (such as color, boiling point, etc) will also be the same. Physical changes involve moving molecules around, but not changing them. Some types of physical changes include:
or formed. This means that the same types of compounds or elements that were there at the beginning of the change are there at the end of the change. Because the ending materials are the same as the beginning materials, the properties (such as color, boiling point, etc) will also be the same. Physical changes involve moving molecules around, but not changing them. Some types of physical changes include:
- Changes of state (changes from a solid to a liquid or a gas and vice versa)
- Separation of a mixture
- Physical deformation (cutting, denting, stretching)
- Making solutions (special kinds of mixtures)
 When we heat the liquid water, it changes to water vapor. But even though the physical properties have changed, the molecules are exactly the same as before. We still have each water molecule containing two hydrogen atoms and one oxygen atom covalently bonded. When you have a jar containing a mixture of pennies and nickels and you sort the mixture so that you have one pile of pennies and another pile of nickels, you have not altered the identity of either the pennies or the nickels - you've merely separated them into two groups. This would be an example of a physical change. Similarly, if you have a piece of paper, you don't change it into something other than a piece of paper by ripping it up. What was paper before you started tearing is still paper when you're done. Again, this is an example of a physical change.
When we heat the liquid water, it changes to water vapor. But even though the physical properties have changed, the molecules are exactly the same as before. We still have each water molecule containing two hydrogen atoms and one oxygen atom covalently bonded. When you have a jar containing a mixture of pennies and nickels and you sort the mixture so that you have one pile of pennies and another pile of nickels, you have not altered the identity of either the pennies or the nickels - you've merely separated them into two groups. This would be an example of a physical change. Similarly, if you have a piece of paper, you don't change it into something other than a piece of paper by ripping it up. What was paper before you started tearing is still paper when you're done. Again, this is an example of a physical change.
For the most part, physical changes tend to be reversible - in other words, they can occur in both directions. You can turn liquid water into solid water through cooling; you can also turn solid water into liquid water through heating. However, as we will later learn, some chemical changes can also be reversed.
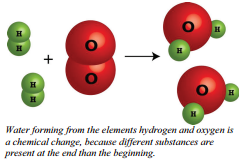 Chemical changes occur when bonds are broken and/or formed between molecules or atoms. This means that one substance with a certain set of properties (such as melting point, color, taste, etc) is turned into a different substance with different properties. Chemical changes are frequently harder to reverse than physical changes.
Chemical changes occur when bonds are broken and/or formed between molecules or atoms. This means that one substance with a certain set of properties (such as melting point, color, taste, etc) is turned into a different substance with different properties. Chemical changes are frequently harder to reverse than physical changes.
One good example of a chemical change is burning paper. In contrast to the act of ripping paper, the act of burning paper actually results in the formation of new chemicals (carbon dioxide and water, to be exact). Another example of chemical change occurs when water is formed. Each molecule contains two atoms of hydrogen and one atom of oxygen chemically bonded.
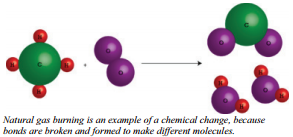

Another example of a chemical change is what occurs when natural gas is burned in your furnace. This time, on the left we have a molecule of methane, \(\ce{CH_4}\), and two molecules of oxygen, \(\ce{O_2}\), while on the right we have two molecules of water, \(\ce{H_2O}\), and one molecule of carbon dioxide, \(\ce{CO_2}\). In this case, not only has the appearance changed, but the structure of the molecules has also changed. The new substances do not have the same chemical properties as the original ones. Therefore, this is a chemical change.
Evidence of Chemical Change
We can't actually see molecules breaking and forming bonds, although that's what defines chemical changes. We have to make other observations to indicate that a chemical change has happened. Some of the evidence for chemical change will involve the energy changes that occur in chemical changes, but some evidence involves the fact that new substances with different properties are formed in a chemical change.
Observations that help to indicate chemical change include:
- Temperature changes (either the temperature increases or decreases)
- Light is given off
- Unexpected color changes (a substance with a different color is made, rather than just mixing the original colors together)
- Bubbles are formed (but the substance is not boiling - you made a substance that is a gas at the temperature of the beginning materials, instead of a liquid)
- Different smell or taste (do not taste your chemistry experiments, though!)
- A solid forms if two clear liquids are mixed (look for floaties - technically called a precipitate)
Example 7.1.1
Label each of the following changes as a physical or chemical change. Give evidence to support your answer.
- Boiling water
- A nail rusting
- A green solution and colorless solution are mixed. The resulting mixture is a solution with a pale green color.
- Two colorless solutions are mixed. The resulting mixture has a yellow precipitate.
Solution:
- Physical: boiling and melting are physical changes. When water boils no bonds are broken or formed. The change could be written: \(\ce{H_2O} \left( l \right) \rightarrow \ce{H_2O} \left( g \right)\)
- Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Color changes indicate chemical change. The following reaction occurs: \(\ce{Fe} + \ce{O_2} \rightarrow \ce{Fe_2O_3}\)
- Physical: because none of the properties changed, this is a physical change. The green mixture is still green and the colorless solution is still colorless. They have just been spread together. No color change occurred or other evidence of chemical change.
- Chemical: the formation of a precipitate and the color change from colorless to yellow indicate a chemical change.
Summary
- Chemists make a distinction between two different types of changes that they study - physical changes and chemical changes.
- Physical changes are changes that do not alter the identity of a substance.
- Chemical changes are changes that occur when one substance is turned into another substance.
- Chemical changes are frequently harder to reverse than physical changes.
- Observations that indicate a chemical change occurred include color change, temperature change, light given off, formation of bubbles, formation of a precipitate, etc.
Vocabulary
- Physical changes: Changes that do not alter the identity of a substance, the same types of molecules are present at the beginning and end of the change.
- Chemical changes: Changes that occur when one substance is turned into another substance; different types of molecules are present at the beginning and end of the change.

