3.3: Valence Electrons
- Page ID
- 49057
Skills to Develop
- Define valence electrons.
- Indicate the number of valence electrons for selected atoms.
The electrons in the outermost shell are the valence electrons. These are the electrons on any atom that can be gained or lost in a chemical reaction. Since filled \(d\) or \(f\) subshells are seldom disturbed in a chemical reaction, the valence electrons include those electrons in the outermost \(s\) and \(p\) sublevels.
Gallium has the following electron configuration:
\[Ga: \: \left[ Ar \right] 4s^2 3d^{10} 4p^1\]
The electrons in the fourth energy level are further from the nucleus than the electrons in the third energy level. The \(4s\) and \(4p\) electrons can be lost in a chemical reaction, but not the electrons in the filled \(3d\) subshell. Gallium therefore has three valence electrons: the two in \(4s\) and one in \(4p\).
Determining Valence Electrons
The number of valence electrons for an atom can be seen in the electron configuration. The electron configuration for magnesium is \(1s^2 2s^2 2p^6 3s^2\). The outer energy level for this atom is \(n = 3\) and it has two electrons in this energy level. Therefore, magnesium has two valence electrons.
The electron configuration for sulfur is \(1s^2 2s^2 2p^6 3s^2 3p^4\). The outer energy level in this atom is \(n = 3\) and it holds six electrons, so sulfur has six valence electrons.
The electron configuration for gallium is \(1s^2 2s^2 2p^6 3s^3 3p^6 4s^2 3d^{10} 4p^1\). The outer energy level for this atom is \(n = 4\) and it contains three electrons. You must recognize that even though the \(3d\) sublevel is mixed in among the \(4s\) and \(4p\) sublevels, \(3d\) is NOT in the outer energy level and therefore, the electrons in the \(3d\) sublevel are NOT valence electrons.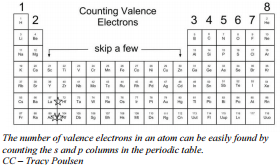 Gallium has three electrons in the outer energy level and therefore, it has three valence electrons. The identification of valence electrons is vital because the chemical behavior of an element is determined primarily by the arrangement of the electrons in the valence shell.
Gallium has three electrons in the outer energy level and therefore, it has three valence electrons. The identification of valence electrons is vital because the chemical behavior of an element is determined primarily by the arrangement of the electrons in the valence shell.
This pattern can be summarized very easily, by merely counting the \(s\) and \(p\) blocks of the periodic table to find the total number of valence electrons. One system of numbering the groups on the periodic table numbers the \(s\) and \(p\) block groups fro 1A to 8A. The number indicates the number of valence electrons.
Summary
- Valence electrons are the electrons in the outermost principal quantum level of an atom.
- The number of valence electrons is important, because the chemical behavior of an element depends primarily by the arrangement of the electrons in the valence shell.
Vocabulary
- Valence electrons: The electrons in the outermost energy level of an atom.

