2.5: The Nature of Light
- Page ID
- 49035
Skills to Develop
- When given two comparative colors or areas in the electromagnetic spectrum, identify which area has the higher wavelength, the higher frequency, and the higher energy.
- Describe the relationship between wavelength, frequency, and energy of light waves (EMR).
Most of us are familiar with waves, whether they are waves of water in the ocean, waves made by wiggling the end of a rope, or waves made when a guitar string is plucked. Light, also called electromagnetic radiation, is a special type of energy that travels as a wave.
Light Energy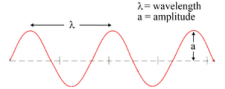
Before we talk about the different forms of light or electromagnetic radiation (EMR), it is important to understand some of the general characteristics that waves share.
The high point of a wave is called the crest. The low point is called the trough. The distance from one point on a wave to the same point on the next wave is called the wavelength of the wave. You could determine the wavelength by measuring the distance from one trough to the next or from the top (crest) of one wave to the crest of the next wave. The symbol used for wavelength is the Greek letter lambda, \(\lambda\).
Another important characteristic of waves is called frequency. The frequency of a wave is the number of waves that pass a given point each second. If we choose an exact position along the path of the wave and count how many waves pass the position each second, we would get a value for frequency. Frequency has the units of cycles/\(\text{sec}\) or waves/\(\text{sec}\), but scientists usually just use units of 1/\(\text{sec}\) or Hertz (\(\text{Hz}\)).
All types of light (EMR) travels at the same speed, \(3.00 \times 10^8 \: \text{m/s}\). Because of this, as the wavelength increases (the waves get longer), the frequency decreases (fewer waves pass). On the other hand, as the wavelength decreases (the waves get shorter), the frequency increases (more waves pass).
Electromagnetic waves (light waves) have an extremely wide range of wavelengths, frequencies, and energies. The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The highest energy form of electromagnetic waves is gamma rays and the lowest energy form (that we have named) is radio waves.
On the far right of the figure below are the electromagnetic waves with the highest energy. These waves are called gamma rays and can be quite dangerous in large numbers to living systems. The next lowest energy form of electromagnetic waves is called x-rays. Most of you are familiar with the penetration abilities of these waves. They can also be dangerous to living systems. Next lower in energy are ultraviolet rays. These rays are part of sunshine and rays on the upper end of the ultraviolet range can cause sunburns and eventually skin cancer. The tiny section next in the spectrum is the visible range of light. These are the frequencies (energies) of the electromagnetic spectrum to which the human eye responds. The highest form of visible light energy is violet light, with red light having the lowest energy of all visible light. Even lower in the spectrum, too low in energy to see, are infrared rays and radio waves.
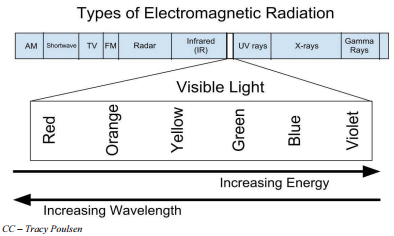
The light energies that are in the visible range are electromagnetic waves that cause the human eye to respond when those frequencies enter the eye. The eye sends signals to the brain and the individual "sees" various colors. The highest energy waves in the visible region cause the brain to see violet and as the energy of the waves decreases, the colors change to blue, green, then to yellow, orange, and red. When the energy of the wave is above or below the visible range, the eye does not respond to them. When the eye receives several different frequencies at the same time, the colors are blended by the brain. If all frequencies of light strike the eye together the brain sees white, and if there are no frequencies striking the eye the brain sees black.
All the objects that you see around you are light absorbers - that is, the chemicals on the surface of the objects absorb certain frequencies and not others. Your eyes detect the frequencies that strike your eye. Therefore, if your friend is wearing a red shirt, it means that the dye in that shirt absorbs every frequency of blue light and the red is reflected. When the red frequency from the shirt strikes your eye, your visual system sees red and you say the shirt is red. If your only light source was one exact frequency of blue light and you shined it on a shirt that absorbed every frequency of light except one exact frequency of red, then the shirt would look black to you because no light would be reflected to your eye. The light from many fluorescent types of light do not contain all the frequencies of sunlight and so clothes inside a store may appear to be a slightly different color than when you get them home.
Summary
- Wave form energy is characterized by velocity, wavelength, and frequency.
- As the wavelength of a wave increases, its frequency decreases. Longer waves with lower frequencies have lower energy. Shorter waves with higher frequencies have higher energy.
- Electromagnetic radiation has a wide spectrum, including low energy radio waves and very high energy gamma rays.
- The different colors of light differ in their frequencies (or wavelengths).
Vocabulary
- Frequency of a wave: The number of waves passing a specific point each second.
- Wavelength: The distance between a point on one wave to the same point on the next wave (usually from crest to crest or trough to trough).
- Electromagnetic spectrum: A list of all the possible types of light in order of decreasing frequency, or increasing wavelength, or decreasing energy. The electromagnetic spectrum includes gamma rays, X-rays, UV rays, visible light, IR radiation, microwaves, and radio waves.

