11.2: Gas Laws
- Page ID
- 52218
Skills to Develop
- Predict effect on pressure, volume, or temperature if one of the other variables are changed.
- Solve problems using the combined gas law.
Gases are often characterized by their volume, temperature, and pressure. These characteristics, however, are not independent of each other. Gas pressure is dependent on the force exerted by the molecular collisions and the area over which the force is exerted. The force exerted by the molecular collisions is dependent on the absolute temperature and so forth. The relationships between these characteristics can be determined both experimentally and logically from their mathematical definitions.
The gas laws are mathematical relationships that exist for gases between the volume, pressure, temperature, and quantity of gas present. They were determined experimentally over a period of 100 years. They are logically derivable from our present day definitions of pressure, volume, and temperature.
Boyle's Law: Pressure vs. Volume
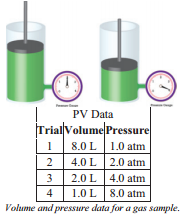 The relationship between pressure and volume of a gas was first determined experimentally by an Irish chemist named Robert Boyle (1627-1691). The relationship between the pressure and volume of a gas is commonly referred to as Boyle's Law.
The relationship between pressure and volume of a gas was first determined experimentally by an Irish chemist named Robert Boyle (1627-1691). The relationship between the pressure and volume of a gas is commonly referred to as Boyle's Law.
When we wish to observe the relationship between two variables, it is absolutely necessary to keep all other variables constant so that the change in one variable can be directly related to the change in the other. Therefore, when the relationship between gas volume and gas pressure is investigated, the quantity of gas and its temperature do not contribute to any observed changes.
You may have noticed that when you try to squeeze a balloon, the resistance to squeezing is greater and the balloon becomes smaller. That is, the pressure inside the balloon becomes greater when the volume is reduced. This phenomenon can be studied more carefully with an apparatus like that in the figure. This is a cylinder tightly fitted with a piston that can be raised or lowered. There is also a pressure gauge fitted to the cylinder so that the gas pressure inside the cylinder can be measured. The amount of gas inside the cylinder cannot change and the temperature of the gas is not allowed to change.
In the picture on the right, the volume of the gas is \(4.0 \: \text{L}\) and the pressure exerted by the gas is \(2.0 \: \text{atm}\). If the piston is pushed down to decrease the volume of the gas to \(2.0 \: \text{L}\), the pressure of the gas is found to increase to \(4.0 \: \text{atm}\). The piston can be moved up and down to positions for several different volumes and the pressure of the gas read at each of the volumes.
We might note from casual observation of the data that doubling volume is associated with the pressure being reduced to half and if we move the piston to cause the pressure to double, the volume is halved. The data show that the relationship is an inverse relationship, meaning that as volume increases the pressure decreases. The opposite is also true.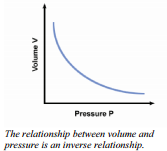
Boyle's Law can be summarized in the following equation:
\[P_1V_1 = P_2V_2\]
Where:
- \(P_1 =\) the initial pressure
- \(V_1 =\) the initial volume
- \(P_2 =\) the final pressure
- \(V_2 =\) the final volume
For this equation, the units used for pressure are unimportant, as long as both pressures have the same unit (either \(\text{mm} \: \ce{Hg}\) or \(\text{atm}\)) and each volume has the same unit (either \(\text{mL}\) or \(\text{L}\)).
Charles's Law: Temperature and Volume
The relationship between the volume and temperature of a gas was investigated by a French physicist, Jacques Charles (1746 - 1823). (As a piece of trivia, Charles was also the first person to fill a large balloon with hydrogen gas and take a solo balloon flight.) The relationship between the volume and temperature of a gas is often referred to as Charles's Law.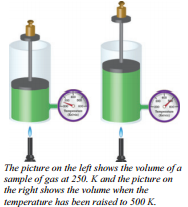
An apparatus that can be used to study the relationship between the temperature and volume of a gas is shown in the picture to the right. Once again, we have a sample of gas trapped inside a cylinder so no gas can get in or out. Thus we have a constant mass of gas. We also have a mass set on top of a moveable piston to keep a constant force pushing against the gas. This guarantees that the gas pressure in the cylinder will be constant because if the pressure inside increases, the piston will be pushed up expanding the inside volume until the inside pressure becomes equal to the outside pressure again.
Similarly, if the inside pressure decreases, the outside pressure will push the cylinder down, decreasing volume, until the two pressures again become the same. This system guarantees constant gas pressure inside the cylinder.
This relationship is a direct relationship. If the temperature, in Kelvin, doubles, so does the 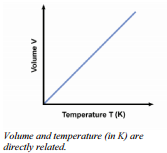 volume. This relationship would also be expected when we recognize that we are increasing the total force of molecular collisions with the walls by raising the temperature and the only way to keep the pressure from increasing is to increase the area over which that larger force is exerted. This mathematical relationship is known as direct proportionality. When one variable is increased, the other variable also increases by exactly the same factor. An equation to show how these values are related is given by:
volume. This relationship would also be expected when we recognize that we are increasing the total force of molecular collisions with the walls by raising the temperature and the only way to keep the pressure from increasing is to increase the area over which that larger force is exerted. This mathematical relationship is known as direct proportionality. When one variable is increased, the other variable also increases by exactly the same factor. An equation to show how these values are related is given by:
\[\frac{V_1}{V_2} = \frac{T_1}{T_2}\]
This relationship is ONLY true if the temperature is measured in Kelvin. However, the units of volume are irrelevant, as long as the two volumes are measured in the same units.
 Gay-Lussac's Law: Temperature and Pressure
Gay-Lussac's Law: Temperature and Pressure
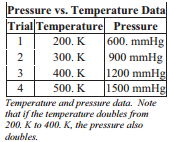
The relationship between temperature and pressure was investigated by the French chemist, Joseph Gay-Lussac (1778 - 1850). In an apparatus used for this investigation, the cylinder does not have a moveable piston because it is necessary to hold the volume constant as well as the quantity of gas. This apparatus allows us to alter the temperature of a gas and measure the pressure exerted by the gas at each temperature.
After a series of temperatures and pressures have been measured, a data table like the others can 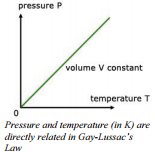 be produced.
be produced.
Temperature and pressure are also directly related, meaning that if the temperature, in Kelvin, doubles, so does the pressure. This relationship is also logical since by increasing temperature, we are increasing the force of molecular collision and keeping the area over which the force is exerted constant requires that the pressure increases.
\[\frac{P_1}{P_2} = \frac{T_1}{T_2}\]
Standard Temperature and Pressure (STP)
It should be apparent by now that expressing a quantity of gas simply by stating its volume is totally inadequate. Ten liters of oxygen gas could contain any mass of oxygen from \(4000 \: \text{g}\) to \(0.50 \: \text{g}\), depending on the temperature and pressure of the gas. Chemists found it useful to have a standard temperature and pressure with which to express gas volume. The standard conditions of temperature and pressure (STP) were chosen to be \(0^\text{o} \text{C}\) \(\left( 273 \: \text{K} \right)\) and \(1.00 \: \text{atm}\) \(\left( 760 \: \text{mm} \: \ce{Hg} \right)\). You will commonly see gas volumes expressed as \(1.5 \: \text{L}\) at STP. Once you know the temperature and pressure conditions of a volume of gas, you can calculate the volume at other conditions and you can also calculate the mass of the gas if you know the formula.
The Combined Gas Law
Boyle's Law shows how the volume of a gas changes when its pressure is changed (temperature held constant) and Charles's Law shows how the volume of a gas changes when the temperature is changed (pressure held constant). Is there a formula we can use to calculate the change in volume of a gas if both pressure and temperature change? The answer is "yes", we can use a formula that combines Boyle's Law and Charles's Law.
This equation is most commonly written in the form shown below and is known as the Combined Gas Law.
\[\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}\]
As in the other laws, when solving problems with the combined gas law, temperatures must always be in Kelvin. The units for pressure and volume may be any appropriate units but the units for each value of pressure must be the same and the units for each value of volume must be the same.
Another interesting point about the combined gas law is that all the other gas laws (Charles's, Gay-Lussac's, and Boyle's) can be derived from this equation. To do this, you simply cancel out the variable that was held constant in the reaction. For example, temperature is constant in Boyle's Law. If you cancel the temperatures out of the Combined Gas Law, you get:
\[P_1V_1 = P_2V_2\]
Although the other equations are not as obvious, the same method can be used to derive the other equations. If you are able to derive the other equations, you will not have to memorize them.
Example 11.2.1
A sample of gas has a volume of 400 liters when its temperature is \(20^\text{o} \text{C}\) and its pressure is \(300 \: \text{mm} \: \ce{Hg}\). What volume will the gas occupy at STP?
Solution:
Step 1: Identify the given information & check units. Temperature must be in Kelvin. Volume units must match and pressure units must match.
\(P_1 = 300 \: \text{mm} \: \ce{Hg}\)
\(V_1 = 400 \: \text{L}\)
\(T_1 = 293 \: \text{K}\) (remember, ALL temperatures must be in Kelvin)
\(P_2 = 760 \: \text{mm} \: \ce{Hg}\)
\(V_2 = ?\)
\(T_2 = 273 \: \text{K}\)
Step 2: Solve the combined gas law for the unknown variable.
\[\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}\]
\[\frac{\left( 300 \: \text{mm} \: \ce{Hg} \right) \left( 400 \: \text{L} \right)}{293 \: \text{K}} = \frac{\left( 769 \: \text{mm} \: \ce{Hg} \right) V_2}{273 \: \text{K}}\]
\(V_2 = 147 \: \text{L}\)
Example 11.2.2
A sample of gas occupies \(1.00 \: \text{L}\) under standard conditions. What temperature would be required for this sample of gas to occupy \(1.50 \: \text{L}\) and exert a pressure of \(2.00 \: \text{atm}\)?
Solution:
Step 1: Identify the given information & check units. Temperature must be in Kelvin. Volume units must match and pressure units must match.
\(P_1 = 1.00 \: \text{atm}\) (standard pressure)
\(V_1 = 1.00 \: \text{L}\)
\(T_1 = 273 \: \text{K}\) (standard temperature, remember, ALL temperatures must be in Kelvin)
\(P_2 = 2.00 \: \text{atm}\)
\(V_2 = 1.50 \: \text{L}\)
\(T_2 = ?\)
Step 2: Solve the combined gas law for the unknown variable.
\[\frac{\left( 1.00 \: \text{atm} \right) \left( 1.00 \: \text{L} \right)}{273 \: \text{K}} = \frac{\left( 2.00 \: \text{atm} \right) \left( 1.50 \: \text{L} \right)}{T_2}\]
\(T_2 = 819 \: \text{K}\)
Example 11.2.3
A sample of gas has a volume of \(500 \: \text{mL}\) under a pressure of \(500 \: \text{mm} \: \ce{Hg}\). What will be the new volume of the gas if the pressure is reduced to \(300 \: \text{mm} \: \ce{Hg}\) at a constant temperature?
Solution:
Step 1: Identify the given information & check units. Temperature must be in Kelvin. Volume units must match and pressure units must match.
\(P_1 = 500 \: \text{mm} \: \ce{Hg}\)
\(V_1 = 500 \: \text{mL}\)
\(P_2 = 300 \: \text{mm} \: \ce{Hg}\)
\(V_2 = ?\)
Temperature is constant, so it cancels out of the combined gas law.
Step 2: Solve the combined gas law for the unknown variable. (Or, recognize this is Boyle's Law and start with that equation.)
\[P_1V_1 = P_2V_2\]
\[\left( 500 \: \text{mm} \: \ce{Hg} \right) \left( 500 \: \text{mL} \right) = \left( 300 \: \text{mm} \: \ce{Hg} \right) V_2\]
\(V_2 = 833 \: \text{mL}\)
Avogadro's Law
Avogadro's Law was known as Avogadro's hypothesis for the first century of its existence. Since Avogadro's hypothesis can now be demonstrated mathematically, it was decided that it should be called a law instead of a hypothesis. Avogadro's Law postulates that equal volumes of gas under the same conditions of temperature and pressure contain the same number of molecules.
This relationship is important for a couple of reasons. It means that all gases under the same conditions behave the same way: all of these equations work equally well for carbon dioxide, helium, or a mixture of gases. Furthermore, we will be able to use this relationship again when we deal with balanced reactions. The volume of two gases at the same temperature and pressure are directly related to the number of molecules (or moles) of the gases involved in a chemical reaction.
Lesson Summary
- For a fixed sample of ideal gas at constant temperature, volume is inversely proportional to pressure.
- For a fixed sample of ideal gas at constant pressure, volume is directly proportional to temperature.
- For a fixed sample of ideal gas at constant volume, pressure is directly proportional to temperature.
- The volume of a mass of gas is dependent on the temperature and pressure. Therefore, these conditions of temperature and pressure are \(0^\text{o} \text{C}\) and \(1.0 \: \text{atm}\).
- Avogadro's Law: Equal volumes of gases under the same conditions of temperature and pressure contain equal numbers of molecules.
Further Reading/Supplemental Links
- Section 7-6 is on the Combined Gas Law. http://www.fordhamprep.org/gcurran/s.../Section31.htm
- http://en.wikipedia.org/wiki/Kinetic_theory
- http://www.chm.davidson.edu/chemistr...cconcepts.html

