4.5: Other Practical Matters in Reactions
- Page ID
- 19955
The Learning Objective of this Module is to understand and quantify incomplete reactions.
When reactants are not present in stoichiometric quantities, the limiting reactant determines the maximum amount of product that can be formed from the reactants. The amount of product calculated in this way is the theoretical yield, the amount obtained if the reaction occurred perfectly and the purification method were 100% efficient.
In reality, less product is always obtained than is theoretically possible because of mechanical losses (such as spilling), separation procedures that are not 100% efficient, competing reactions that form undesired products, and reactions that simply do not run to completion, resulting in a mixture of products and reactants; this last possibility is a common occurrence. Therefore, the actual yield, the measured mass of products obtained from a reaction, is almost always less than the theoretical yield (often much less). The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage:
\[ percent \, yield = {actual \, yield \, (g) \over theoretical \, yield \, (g) } \times 100 \tag{3.23} \]
The method used to calculate the percent yield of a reaction is illustrated in Example 13.
| Example 13 |
|---|
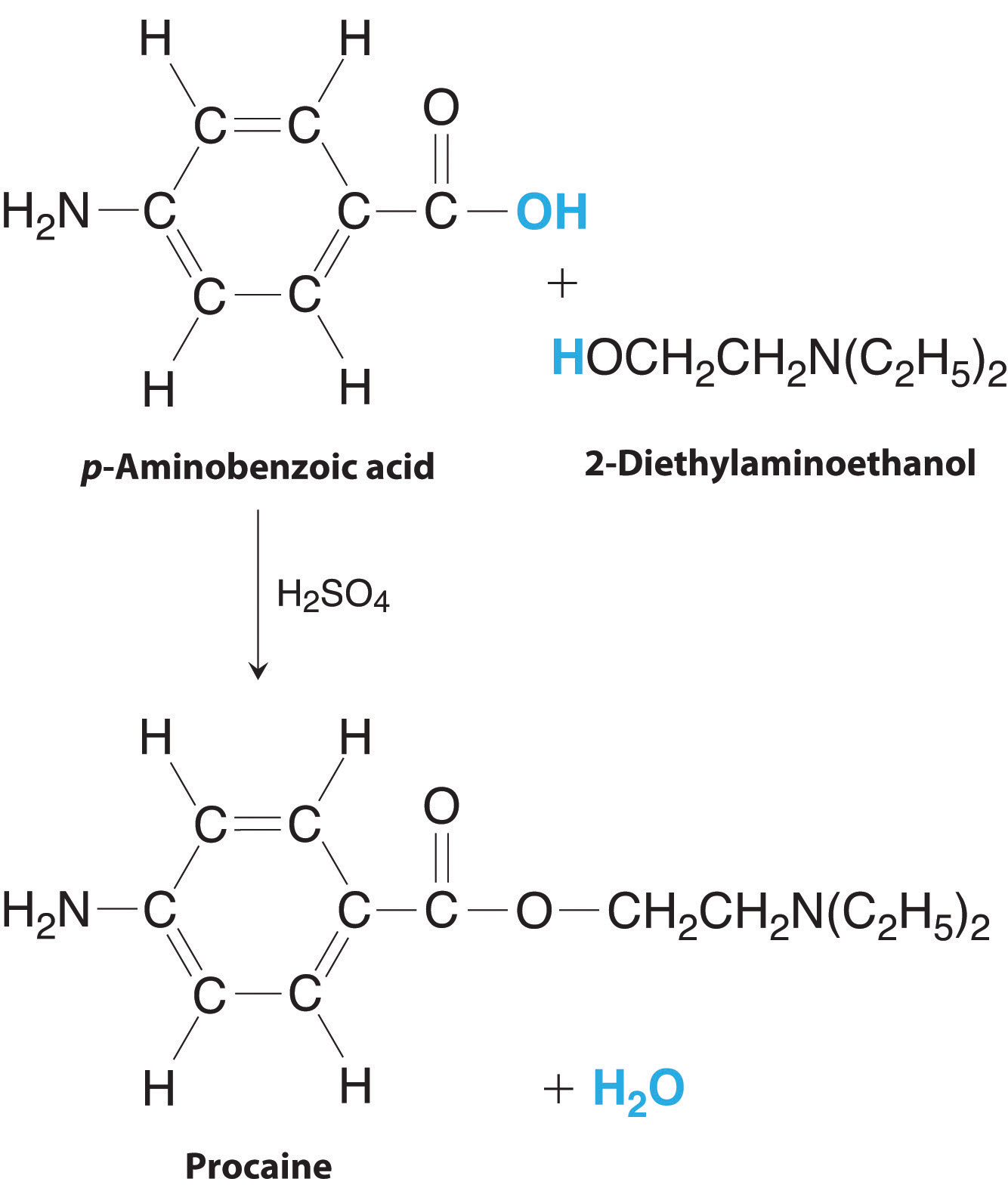
| Procaine is a key component of Novocain, an injectable local anesthetic used in dental work and minor surgery. Procaine can be prepared in the presence of H2SO4 (indicated above the arrow) by the reaction \[ \underset {p-amino benzoic\,acid}{C_7H_7NO_2} + \underset {2-diethylaminoethanol}{C_6H_{15}NO}\,\,\underrightarrow {H_2SO_4} \,\, \underset {procaine}{C_{13}H_{20}N_2O_2} + H_2O\] If this reaction was carried out using 10.0 g of p-aminobenzoic acid and 10.0 g of 2-diethylaminoethanol, and 15.7 g of procaine were isolated, what is the percent yield?
The preparation of procaine. A reaction of p-aminobenzoic acid with 2-diethylaminoethanol yields procaine and water. Given: masses of reactants and product Asked for: percent yield Strategy:
Solution: A From the formulas given for the reactants and the products, we see that the chemical equation is balanced as written. According to the equation, 1 mol of each reactant combines to give 1 mol of product plus 1 mol of water. B To determine which reactant is limiting, we need to know their molar masses, which are calculated from their structural formulas: p-aminobenzoic acid (C7H7NO2), 137.14 g/mol; 2-diethylaminoethanol (C6H15NO), 117.19 g/mol. Thus the reaction used the following numbers of moles of reactants: \[ moles \, p-aminobenzoic \, acid = 10.0 \, g \, \times \, {1 \, mol \over 137.14 \, g } = 0.0729 \, mol \, p-aminbenzoic\, acid \] \[ moles \, 2-diethylaminoethanol = 10.0 \, g \times {1 \, mol \over 117.19 \, g} = 0.0853 \, mol \, 2-diethylaminoethanol \] \[ theoretical \, yield \, of \, procaine = 0.0729 \, mol \times {236.31 \, g \over 1 \, mol } = 17.2 \, g \] C The actual yield was only 15.7 g of procaine, so the percent yield is \[ percent \, yield = {15.7 \, g \over 17.2 \, g } \times 100 = 91.3 \% \] (If the product were pure and dry, this yield would indicate very good lab technique!) |
| Exercise |
|---|
| Lead was one of the earliest metals to be isolated in pure form. It occurs as concentrated deposits of a distinctive ore called galena (PbS), which is easily converted to lead oxide (PbO) in 100% yield by roasting in air via the following reaction: \[ 2PbS (s) + 3O_2 \rightarrow 2PbO (s) + 2SO_2 (g) \] The resulting PbO is then converted to the pure metal by reaction with charcoal. Because lead has such a low melting point (327°C), it runs out of the ore-charcoal mixture as a liquid that is easily collected. The reaction for the conversion of lead oxide to pure lead is as follows: \[ PbO (s) + C(s) \rightarrow Pb (l) + CO (g) \] If 93.3 kg of PbO is heated with excess charcoal and 77.3 kg of pure lead is obtained, what is the percent yield?
Electrolytically refined pure (99.989 %) superficially oxidized lead nodules and a high purity (99.989 %) \(1\; cm^3\) lead cube for comparison. Figure used with permission from Wikipedia. Answer: 89.2% |
Percent yield can range from 0% to 100%. In the laboratory, a student will occasionally obtain a yield that appears to be greater than 100%. This usually happens when the product is impure or is wet with a solvent such as water. If this is not the case, then the student must have made an error in weighing either the reactants or the products. The law of conservation of mass applies even to undergraduate chemistry laboratory experiments. A 100% yield means that everything worked perfectly, and the chemist obtained all the product that could have been produced. Anyone who has tried to do something as simple as fill a salt shaker or add oil to a car’s engine without spilling knows the unlikelihood of a 100% yield. At the other extreme, a yield of 0% means that no product was obtained. A percent yield of 80%–90% is usually considered good to excellent; a yield of 50% is only fair. In part because of the problems and costs of waste disposal, industrial production facilities face considerable pressures to optimize the yields of products and make them as close to 100% as possible.
Summary
The stoichiometry of a reaction describes the relative amounts of reactants and products in a balanced chemical equation. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant(s). If a quantity of a reactant remains unconsumed after complete reaction has occurred, it is in excess. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the reaction. The actual yield is the amount of product(s) actually obtained in the reaction; it cannot exceed the theoretical yield. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, expressed as a percentage.
Key Takeaway
The stoichiometry of a balanced chemical equation identifies the maximum amount of product that can be obtained.
| Conceptual Problems |
|---|
|
| Numerical Problems |
|---|
| Please be sure you are familiar with the topics discussed in Essential Skills 2 () before proceeding to the Numerical Problems. 17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.) a. b. Cu(s) + H2SO4(aq) → CuSO4(aq) + SO2(g) + H2O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide c. AgC2H3O2(aq) + Na3PO4(aq) → Ag3PO4(s) + NaC2H3O2(aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate 18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction? 19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction? 20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting. A + 3B → 2C 21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol. a. Write a balanced chemical equation for this reaction. b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide. 22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction: NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq) a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO2? b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide. 23. The reaction of propane gas (CH3CH2CH3) with chlorine gas (Cl2) produces two monochloride products: CH3CH2CH2Cl and CH3CHClCH3. The first is obtained in a 43% yield and the second in a 57% yield. a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion? b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane? c. Use the actual percent yield to calculate how many grams of each product would actually be obtained. 24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions: \[ 2PaI_5 (s) \underrightarrow {\Delta} 2Pa (s) + 5I_2 (s) \] a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized? b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction? c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation? 25. Aniline (C6H5NH2) can be produced from chlorobenzene (C6H5Cl) via the following reaction: C6H5Cl(l) + 2NH3(g) → C6H5NH2(l) + NH4Cl(s) Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia. a. Which is the limiting reactant? b. Which reactant is present in excess? c. What is the theoretical yield of ammonium chloride in grams? d. If 4.78 g of NH4Cl was recovered, what was the percent yield? e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess. 26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following: a. 9.36 × 1024 formula units of NaCl b. 8.5 × 104 mol of Br2 c. 3.7 × 108 g of NaCl |
| Answers |
|---|
| 17. a. 80% b. 30% c. 35.7% 19. 45%. 21. a. CO + 2H2 → CH3OH b. 58.28% 23. a. 2.24 g Cl2 b. 4.95 g c. 2.13 g CH3CH2CH2Cl plus 2.82 g CH3CHClCH3 25. a. chlorobenzene b. ammonia c. 8.74 g ammonium chloride. d. 55% e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \) |