4.2: Chemical Equations and Stoichiometry
- Page ID
- 19231
The Learning Objective of this Module is to calculate the quantities of compounds produced or consumed in a chemical reaction.
A balanced chemical equation gives the identity of the reactants and the products as well as the accurate number of molecules or moles of each that are consumed or produced. Stoichiometry is a collective term for the quantitative relationships between the masses, the numbers of moles, and the numbers of particles (atoms, molecules, and ions) of the reactants and the products in a balanced chemical equation. A stoichiometric quantity is the amount of product or reactant specified by the coefficients in a balanced chemical equation. In , for example, the stoichiometry of the reaction for the ammonium dichromate volcano was expressed in terms of the atoms, ions, or molecules involved and the numbers of moles, grams, and formula units of each (recognizing, for instance, that 1 mol of ammonium dichromate produces 4 mol of water). This section describes how to use the stoichiometry of a reaction to answer questions like the following: How much oxygen is needed to ensure complete combustion of a given amount of isooctane? (This information is crucial to the design of nonpolluting and efficient automobile engines.) How many grams of pure gold can be obtained from a ton of low-grade gold ore? (The answer determines whether the ore deposit is worth mining.) If an industrial plant must produce a certain number of tons of sulfuric acid per week, how much elemental sulfur must arrive by rail each week?
All these questions can be answered using the concepts of the mole and molar and formula masses, along with the coefficients in the appropriate balanced chemical equation.
Stoichiometry Problems
When carrying out a reaction in either an industrial setting or a laboratory, it is easier to work with masses of substances than with the numbers of molecules or moles. The general method for converting from the mass of any reactant or product to the mass of any other reactant or product using a balanced chemical equation is outlined in and described in the following text.
Steps in Converting between Masses of Reactant and Product
- Convert the mass of one substance (substance A) to the corresponding number of moles using its molar mass.
- From the balanced chemical equation, obtain the number of moles of another substance (B) from the number of moles of substance A using the appropriate mole ratio (the ratio of their coefficients).
- Convert the number of moles of substance B to mass using its molar mass. It is important to remember that some species are in excess by virtue of the reaction conditions. For example, if a substance reacts with the oxygen in air, then oxygen is in obvious (but unstated) excess.
Converting amounts of substances to moles—and vice versa—is the key to all stoichiometry problems, whether the amounts are given in units of mass (grams or kilograms), weight (pounds or tons), or volume (liters or gallons).
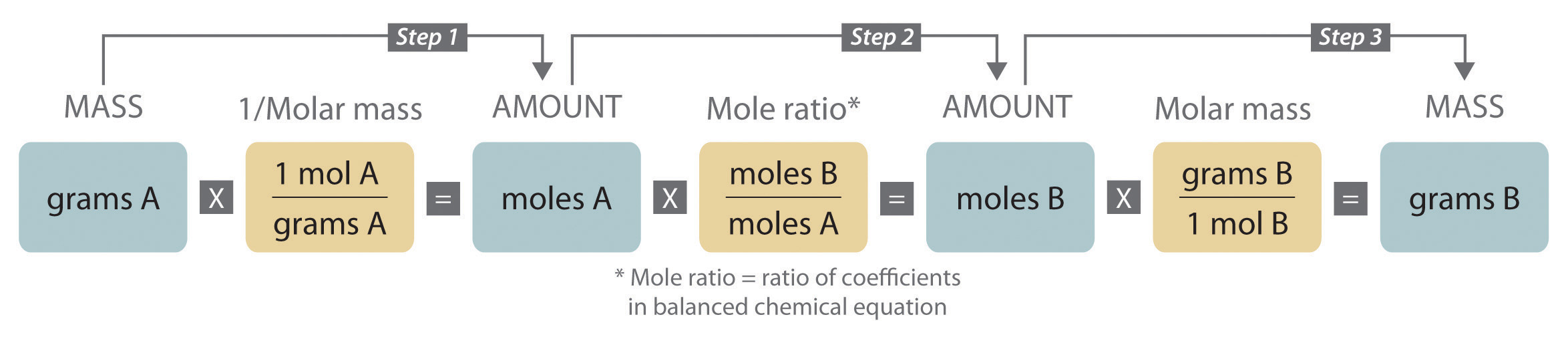
Figure 3.11 A Flowchart for Stoichiometric Calculations Involving Pure Substances. The molar masses of the reactants and the products are used as conversion factors so that you can calculate the mass of product from the mass of reactant and vice versa.
To illustrate this procedure, consider the combustion of glucose. Glucose reacts with oxygen to produce carbon dioxide and water:
\[ C_6H_{12}O_6 (s) + 6 O_2 (g) \rightarrow 6 CO_2 (g) + 6 H_2O (l) \tag{3.20}\]
Just before a chemistry exam, suppose a friend reminds you that glucose is the major fuel used by the human brain. You therefore decide to eat a candy bar to make sure that your brain does not run out of energy during the exam (even though there is no direct evidence that consumption of candy bars improves performance on chemistry exams). If a typical 2 oz candy bar contains the equivalent of 45.3 g of glucose and the glucose is completely converted to carbon dioxide during the exam, how many grams of carbon dioxide will you produce and exhale into the exam room?
The initial step in solving a problem of this type is to write the balanced chemical equation for the reaction. Inspection shows that it is balanced as written, so the strategy outlined in , can be adapted as follows:
1. Use the molar mass of glucose (to one decimal place, 180.2 g/mol) to determine the number of moles of glucose in the candy bar:
\[ moles \, glucose = 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose } = 0.251 \, mol \, glucose \]
2. According to the balanced chemical equation, 6 mol of CO2 is produced per mole of glucose; the mole ratio of CO2 to glucose is therefore 6:1. The number of moles of CO2 produced is thus
\[ moles \, CO_2 = mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \]
\[ = 0.251 \, mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \]
\[ = 1.51 \, mol \, CO_2 \]
These operations can be summarized as follows:
\[ 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose} \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose} \times {44.010 \, g \, CO_2 \over 1 \, mol \, CO_2} = 66.4 \, g \, CO_2 \]
Discrepancies between the two values are attributed to rounding errors resulting from using stepwise calculations in steps 1–3. (For more information about rounding and significant digits, see Essential Skills 1 in , .) This amount of gaseous carbon dioxide occupies an enormous volume—more than 33 L. Similar methods can be used to calculate the amount of oxygen consumed or the amount of water produced.
The balanced chemical equation was used to calculate the mass of product that is formed from a certain amount of reactant. It can also be used to determine the masses of reactants that are necessary to form a certain amount of product or, as shown in Example 11, the mass of one reactant that is required to consume a given mass of another reactant.
| Example 11 |
|---|
| The combustion of hydrogen with oxygen to produce gaseous water is extremely vigorous, producing one of the hottest flames known. Because so much energy is released for a given mass of hydrogen or oxygen, this reaction was used to fuel the NASA (National Aeronautics and Space Administration) space shuttles, which have recently been retired from service. NASA engineers calculated the exact amount of each reactant needed for the flight to make sure that the shuttles did not carry excess fuel into orbit. Calculate how many tons of hydrogen a space shuttle needed to carry for each 1.00 tn of oxygen (1 tn = 2000 lb). The US space shuttle Discovery during liftoff. The large cylinder in the middle contains the oxygen and hydrogen that fueled the shuttle’s main engine. Given: reactants, products, and mass of one reactant Asked for: mass of other reactant Strategy:
Solution: We use the same general strategy for solving stoichiometric calculations as in the preceding example. Because the amount of oxygen is given in tons rather than grams, however, we also need to convert tons to units of mass in grams. Another conversion is needed at the end to report the final answer in tons. A We first use the information given to write a balanced chemical equation. Because we know the identity of both the reactants and the product, we can write the reaction as follows: \[ H_2 (g) + O_2 (g) \rightarrow H_2O (g) \] This equation is not balanced because there are two oxygen atoms on the left side and only one on the right. Assigning a coefficient of 2 to both H2O and H2 gives the balanced chemical equation: \[ 2 H_2 (g) + O_2 (g) \rightarrow 2 H_2O (g) \] Thus 2 mol of H2 react with 1 mol of O2 to produce 2 mol of H2O. 1. B To convert tons of oxygen to units of mass in grams, we multiply by the appropriate conversion factors: \[ mass \, of \, O_2 = 1.00 \, tn \times { 2000 \, lb \over tn} \times {453.6 \, g \over lb} = 9.07 \times 10^5 \, g \, O_2 \] Using the molar mass of O2 (32.00 g/mol, to four significant figures), we can calculate the number of moles of O2 contained in this mass of O2: \[ mol \, O_2 = 9.07 \times 10^5 \, g \, O_2 \times {1 \, mol \, O_2 \over 32.00 \, g \, O_2} = 2.83 \times 10^4 \, mol \, O_2 \] 2. Now use the coefficients in the balanced chemical equation to obtain the number of moles of H2 needed to react with this number of moles of O2: \[ mol \, H_2 = mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} \] \[ = 2.83 \times 10^4 \, mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} = 5.66 \times 10^4 \, mol \, H_2 \] 3. The molar mass of H2 (2.016 g/mol) allows us to calculate the corresponding mass of H2: \[mass \, of \, H_2 = 5.66 \times 10^4 \, mol \, H_2 \times {2.016 \, g \, H_2 \over mol \, H_2} = 1.14 \times 10^5 \, g \, H_2 \] Finally, convert the mass of H2 to the desired units (tons) by using the appropriate conversion factors: \[ tons \, H_2 = 1.14 \times 10^5 \, g \, H_2 \times {1 \, lb \over 453.6 \, g} \times {1 \, tn \over 2000 \, lb} = 0.126 \, tn \, H_2 \] The space shuttle had to be designed to carry 0.126 tn of H2 for each 1.00 tn of O2. Even though 2 mol of H2 are needed to react with each mole of O2, the molar mass of H2 is so much smaller than that of O2 that only a relatively small mass of H2 is needed compared to the mass of O2. |
| Exercise 11: Roasting Cinnabar |
|---|
| Cinnabar, (or Cinnabarite) \(HgS\) is the common ore of mercury. Because of its mercury content, cinnabar can be toxic to human beings.
Cinnabar ore. Image used with permission from Wikipedia Alchemists produced elemental mercury by roasting the mercury-containing ore cinnabar (HgS) in air: \[ HgS (s) + O_2 (g) \rightarrow Hg (l) + SO_2 (g) \] The volatility and toxicity of mercury make this a hazardous procedure, which likely shortened the life span of many alchemists. Given 100 g of cinnabar, how much elemental mercury can be produced from this reaction? Answer: 86.2 g |
Summary
The stoichiometry of a reaction describes the relative amounts of reactants and products in a balanced chemical equation. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant(s).
Key Takeaway
The stoichiometry of a balanced chemical equation identifies the maximum amount of product that can be obtained.
Conceptual Problems
- Engineers use conservation of mass, called a “mass balance,” to determine the amount of product that can be obtained from a chemical reaction. Mass balance assumes that the total mass of reactants is equal to the total mass of products. Is this a chemically valid practice? Explain your answer.
- Given the equation \[2H_{2\;(g)} + O_{2\;(g)} \rightarrow 2H+2O_{(g)},\] is it correct to say that 10 g of hydrogen will react with 10 g of oxygen to produce 20 g of water vapor?
- What does it mean to say that a reaction is stoichiometric?
- When sulfur is burned in air to produce sulfur dioxide, what is the limiting reactant? Explain your answer.
- Is it possible for the percent yield to be greater than the theoretical yield? Justify your answer.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Numerical Problems.
1. What is the formula mass of each species?
a. ammonium chloride
b. sodium cyanide
c. magnesium hydroxide
d. calcium phosphate
e. lithium carbonate
f. hydrogen sulfite ion
2. What is the molecular or formula mass of each compound?
a. potassium permanganate
b. sodium sulfate
c. hydrogen cyanide
d. potassium thiocyanate
e. ammonium oxalate
f. lithium acetate
3. How many moles are in each of the following?
a. 10.76 g of Si
b. 8.6 g of Pb
c. 2.49 g of Mg
d. 0.94 g of La
e. 2.68 g of chlorine gas
f. 0.089 g of As
4. How many moles are in each of the following?
a. 8.6 g of CO2
b. 2.7 g of CaO
c. 0.89 g of KCl
d. 4.3 g of SrBr2
e. 2.5 g of NaOH
f. 1.87 g of Ca(OH)2
5. Convert the following to moles and millimoles.
a. 1.68 g of Ba(OH)2
b. 0.792 g of H3PO4
c. 3.21 g of K2S
d. 0.8692 g of Cu(NO3)2
e. 10.648 g of Ba3(PO4)2
f. 5.79 g of (NH4)2SO4
g. 1.32 g of Pb(C2H3O2)2
h. 4.29 g of CaCl2·6H2O
6. Convert the following to moles and millimoles.
a. 0.089 g of silver nitrate
b. 1.62 g of aluminum chloride
c. 2.37 g of calcium carbonate
d. 1.004 g of iron(II) sulfide
e. 2.12 g of dinitrogen pentoxide
f. 2.68 g of lead(II) nitrate
g. 3.02 g of ammonium phosphate
h. 5.852 g of sulfuric acid
i. 4.735 g of potassium dichromate
7. What is the mass of each substance in grams and milligrams?
a. 5.68 mol of Ag
b. 2.49 mol of Sn
c. 0.0873 mol of Os
d. 1.74 mol of Si
e. 0.379 mol of H2
f. 1.009 mol of Zr
8. What is the mass of each substance in grams and milligrams?
a. 2.080 mol of CH3OH
b. 0.288 mol of P4
c. 3.89 mol of ZnCl2
d. 1.800 mol of Fe(CO)5
e. 0.798 mol of S8
f. 4.01 mol of NaOH
9. What is the mass of each compound in kilograms?
a. 6.38 mol of P4O10
b. 2.26 mol of Ba(OH)2
c. 4.35 mol of K3PO4
d. 2.03 mol of Ni(ClO3)2
e. 1.47 mol of NH4NO3
f. 0.445 mol of Co(NO3)3
10. How many atoms are contained in each?
a. 2.32 mol of Bi
b. 0.066 mol of V
c. 0.267 mol of Ru
d. 4.87 mol of C
e. 2.74 g of I2
f. 1.96 g of Cs
g. 7.78 g of O2
11. Convert each number of atoms to milligrams.
a. 5.89 × 1022 Pt atoms
b. 2.899 × 1021 Hg atoms
c. 4.826 × 1022 atoms of chlorine
12. Write a balanced chemical equation for each reaction and then determine which reactant is in excess.
a. 2.46 g barium(s) plus 3.89 g bromine(l) in water to give barium bromide
b. 1.44 g bromine(l) plus 2.42 g potassium iodide(s) in water to give potassium bromide and iodine
c. 1.852 g of Zn metal plus 3.62 g of sulfuric acid in water to give zinc sulfate and hydrogen gas
d. 0.147 g of iron metal reacts with 0.924 g of silver acetate in water to give iron(II) acetate and silver metal
e. 3.142 g of ammonium phosphate reacts with 1.648 g of barium hydroxide in water to give ammonium hydroxide and barium phosphate
13. Under the proper conditions, ammonia and oxygen will react to form dinitrogen monoxide (nitrous oxide, also called laughing gas) and water. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 24.6 g of ammonia and 21.4 g of oxygen
b. 3.8 mol of ammonia and 84.2 g of oxygen
c. 3.6 × 1024 molecules of ammonia and 318 g of oxygen
d. 2.1 mol of ammonia and 36.4 g of oxygen
14. When a piece of zinc metal is placed in aqueous hydrochloric acid, zinc chloride is produced, and hydrogen gas is evolved. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 12.5 g of HCl and 7.3 g of Zn
b. 6.2 mol of HCl and 100 g of Zn
c. 2.1 × 1023 molecules of Zn and 26.0 g of HCl
d. 3.1 mol of Zn and 97.4 g of HCl
15. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. NaI(aq) + Cl2(g) → NaCl(aq) + I2(s); 1.0 mol of NaCl
b. NaCl(aq) + H2SO4(aq) → HCl(g) + Na2SO4(aq); 0.50 mol of HCl
c. NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq); 1.5 mol of HNO3
16. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. AgNO3(aq) + CaCl2(s) → AgCl(s) + Ca(NO3)2(aq); 1.25 mol of AgCl
b. Pb(s) + PbO2(s) + H2SO4(aq) → PbSO4(s) + H2O(l); 3.8 g of PbSO4
c. H3PO4(aq) + MgCO3(s) → Mg3(PO4)2(s) + CO2(g) + H2O(l); 6.41 g of Mg3(PO4)2
17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.)
a.
b. Cu(s) + H2SO4(aq) → CuSO4(aq) + SO2(g) + H2O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide
c. AgC2H3O2(aq) + Na3PO4(aq) → Ag3PO4(s) + NaC2H3O2(aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate
18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction?
19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction?
20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting.
A + 3B → 2C
21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol.
a. Write a balanced chemical equation for this reaction.
b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide.
22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction:
NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq)
a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO2?
b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide.
23. The reaction of propane gas (CH3CH2CH3) with chlorine gas (Cl2) produces two monochloride products: CH3CH2CH2Cl and CH3CHClCH3. The first is obtained in a 43% yield and the second in a 57% yield.
a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion?
b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane?
c. Use the actual percent yield to calculate how many grams of each product would actually be obtained.
24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions:
\[ 2PaI_5 (s) \underrightarrow {\Delta} 2Pa (s) + 5I_2 (s) \]
a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized?
b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction?
c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation?
25. Aniline (C6H5NH2) can be produced from chlorobenzene (C6H5Cl) via the following reaction:
C6H5Cl(l) + 2NH3(g) → C6H5NH2(l) + NH4Cl(s)
Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia.
a. Which is the limiting reactant?
b. Which reactant is present in excess?
c. What is the theoretical yield of ammonium chloride in grams?
d. If 4.78 g of NH4Cl was recovered, what was the percent yield?
e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess.
26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following:
a. 9.36 × 1024 formula units of NaCl
b. 8.5 × 104 mol of Br2
c. 3.7 × 108 g of NaCl
Numerical Answers
1.
a. 53.941 amu
b. 49.0072 amu
c. 58.3197 amu
d. 310.177 amu
e. 73.891 amu
f. 81.071 amu
3.
a. 0.3831 mol Si
b. 4.2 × 10−2 mol Pb
c. 0.102 mol Mg
d. 6.8 × 10−3 mol La
e. 3.78 × 10−2 mol Cl2
f. 1.2 × 10−3 mol As
5.
a. 9.80 × 10−3 mol or 9.80 mmole Ba(OH)2
b. 8.08 × 10−3 mol or 8.08 mmole H3PO4
c. 2.91 × 10−2mol or 29.1 mmole K2S
d. 4.634 × 10−3 mol or 4.634 mmole Cu(NO3)2
e. 1.769 × 10−2 mol 17.69 mmole Ba3(PO4)2
f. 4.38 × 10−2 mol or 43.8 mmole (NH4)2SO4
g. 4.06 × 10−3 mol or 4.06 mmole Pb(C2H3O2)2
h. 1.96 × 10−2 mol or 19.6 mmole CaCl2· 6H2O
7.
a. 613 g or 6.13 × 105 mg Ag
b. 296 g or 2.96 × 105 mg Sn
c. 16.6 g or 1.66 × 104 mg Os
d. 48.9 g or 4.89 × 104 mg Si
e. 0.764 g or 764 mg H2
f. 92.05 g or 9.205 × 104 mg Zr
9.
a. 1.81 kg P4O10
b. 0.387 kg Ba(OH)2
c. 0.923 kg K3PO4
d. 0.458 kg Ni(ClO3)2
e. 0.118 kg (NH4)NO3
f. 0.109 kg Co(NO3)3
11.
a. 1.91 × 104 mg Pt
b. 965.6 mg Hg
c. 2841 mg Cl
13. The balanced chemical equation for this reaction is
2NH3 + 2O2 → N2O + 3H2O
a. NH3
b. NH3
c. O2
d. NH3
15.
a. 150 g NaI and 35 g Cl2
b. 29 g NaCl and 25 g H2SO4
c. 140 g NO2 and 27 g H2O
17.
a. 80%
b. 30%
c. 35.7%
19. 45%.
21.
a. CO + 2H2 → CH3OH
b. 58.28%
23.
a. 2.24 g Cl2
b. 4.95 g
c. 2.13 g CH3CH2CH2Cl plus 2.82 g CH3CHClCH3
25.
a. chlorobenzene
b. ammonia
c. 8.74 g ammonium chloride.
d. 55%
e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \)



