3.7: Saturated Hydrocarbons
- Page ID
- 19749
Approximately one-third of the compounds produced industrially are organic compounds. All living organisms are composed of organic compounds, as are most foods, medicines, clothing fibers, and plastics. Two organic compounds that are commonly encountered are methane (\(CH_4\)) and methanol (\(CH_3OH\)). These and other organic compounds appear frequently in discussions and examples throughout this text.
The detection of organic compounds is useful in many fields. In one recently developed application, scientists have devised a new method called “material degradomics” to monitor the degradation of old books and historical documents. As paper ages, it produces a familiar “old book smell” from the release of organic compounds in gaseous form. The composition of the gas depends on the original type of paper used, a book’s binding, and the applied media. By analyzing these organic gases and isolating the individual components, preservationists are better able to determine the condition of an object and those books and documents most in need of immediate protection.
The simplest class of organic compounds is the hydrocarbons, which consist entirely of carbon and hydrogen. Petroleum and natural gas are complex, naturally occurring mixtures of many different hydrocarbons that furnish raw materials for the chemical industry. The four major classes of hydrocarbons are the following: the alkanes, which contain only carbon–hydrogen and carbon–carbon single bonds; the alkenes, which contain at least one carbon–carbon double bond; the alkynes, which contain at least one carbon–carbon triple bond; and the aromatic hydrocarbons, which usually contain rings of six carbon atoms that can be drawn with alternating single and double bonds. Alkanes are also called saturated hydrocarbons, whereas hydrocarbons that contain multiple bonds (alkenes, alkynes, and aromatics) are unsaturated (not discussed in Chem 2A).
Alkanes
The simplest alkane is methane (CH4), a colorless, odorless gas that is the major component of natural gas. In larger alkanes whose carbon atoms are joined in an unbranched chain (straight-chain alkanes), each carbon atom is bonded to at most two other carbon atoms. The structures of two simple alkanes are shown in Figure 2.15, and the names and condensed structural formulas for the first 10 straight-chain alkanes are in Table 2.7. The names of all alkanes end in -ane, and their boiling points increase as the number of carbon atoms increases.
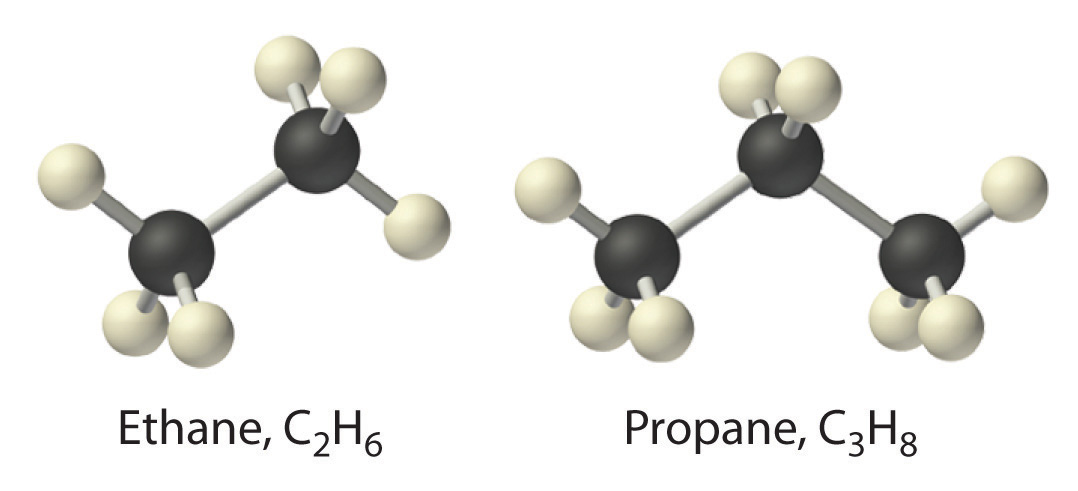
Figure 2.15 Straight-Chain Alkanes with Two and Three Carbon Atoms
Table 2.7 The First 10 Straight-Chain Alkanes
| Name | Number of Carbon Atoms | Molecular Formula | Condensed Structural Formula | Boiling Point (°C) | Uses |
|---|---|---|---|---|---|
| methane | 1 | CH4 | CH4 | −162 | natural gas constituent |
| ethane | 2 | C2H6 | CH3CH3 | −89 | natural gas constituent |
| propane | 3 | C3H8 | CH3CH2CH3 | −42 | bottled gas |
| butane | 4 | C4H10 | CH3CH2CH2CH3 or CH3(CH2)2CH3 | 0 | lighters, bottled gas |
| pentane | 5 | C5H12 | CH3(CH2)3CH3 | 36 | solvent, gasoline |
| hexane | 6 | C6H14 | CH3(CH2)4CH3 | 69 | solvent, gasoline |
| heptane | 7 | C7H16 | CH3(CH2)5CH3 | 98 | solvent, gasoline |
| octane | 8 | C8H18 | CH3(CH2)6CH3 | 126 | gasoline |
| nonane | 9 | C9H20 | CH3(CH2)7CH3 | 151 | gasoline |
| decane | 10 | C10H22 | CH3(CH2)8CH3 | 174 | kerosene |
Alkanes with four or more carbon atoms can have more than one arrangement of atoms. The carbon atoms can form a single unbranched chain, or the primary chain of carbon atoms can have one or more shorter chains that form branches. For example, butane (C4H10) has two possible structures. Normal butane (usually called n-butane) is CH3CH2CH2CH3, in which the carbon atoms form a single unbranched chain. In contrast, the condensed structural formula for isobutane is (CH3)2CHCH3, in which the primary chain of three carbon atoms has a one-carbon chain branching at the central carbon. Three-dimensional representations of both structures are as follows:
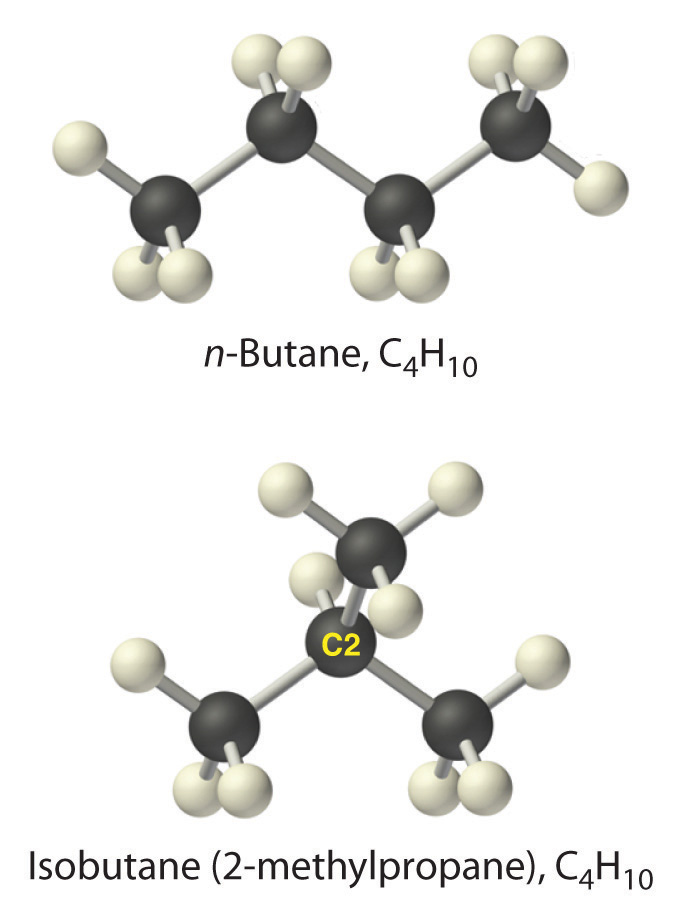
The systematic names for branched hydrocarbons use the lowest possible number to indicate the position of the branch along the longest straight carbon chain in the structure. Thus the systematic name for isobutane is 2-methylpropane, which indicates that a methyl group (a branch consisting of –CH3) is attached to the second carbon of a propane molecule. One of the major components of gasoline is commonly called isooctane; its structure is as follows:
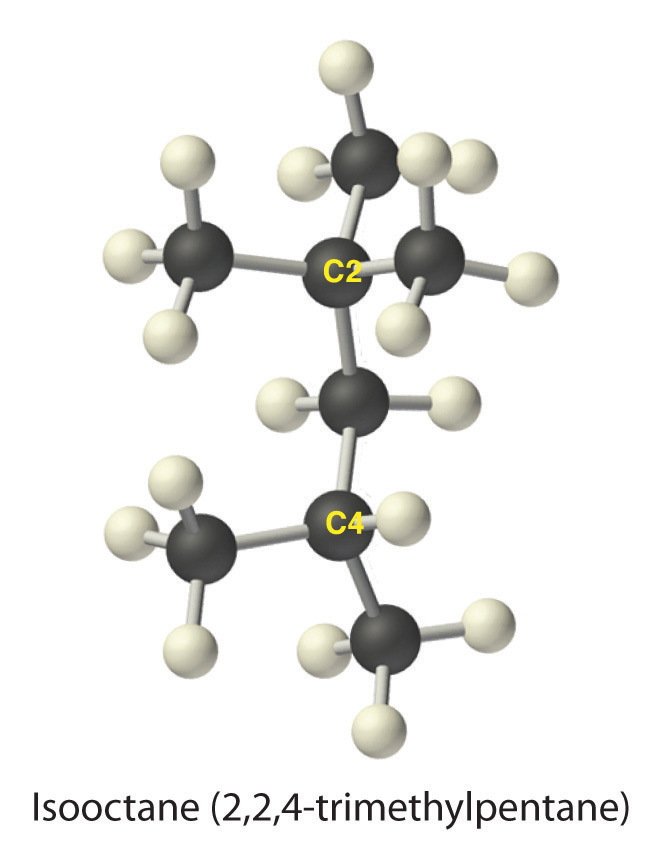
The compound has a chain of five carbon atoms, so it is a derivative of pentane. There are two methyl group branches at one carbon atom and one methyl group at another. Using the lowest possible numbers for the branches gives 2,2,4-trimethylpentane for the systematic name of this compound.

