2.3: The Nuclear Atom
- Page ID
- 19123
The Learning Objective of this module is to become familiar with the components and structure of the atom.
The Atomic Model
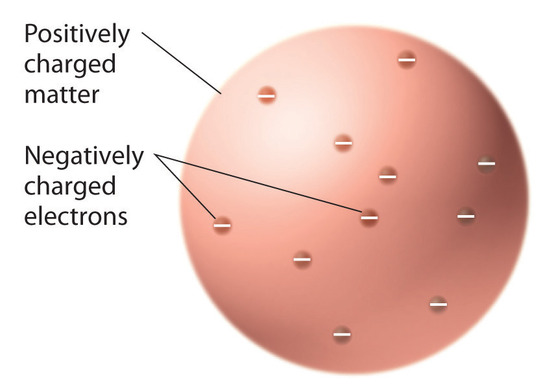
Once scientists concluded that all matter contains negatively charged electrons, it became clear that atoms, which are electrically neutral, must also contain positive charges to balance the negative ones. Thomson proposed that the electrons were embedded in a uniform sphere that contained both the positive charge and most of the mass of the atom, much like raisins in plum pudding or chocolate chips in a cookie (Figure 1.20).
Figure 1.20 Thomson’s Plum Pudding or Chocolate Chip Cookie Model of the Atom. In this model, the electrons are embedded in a uniform sphere of positive charge.
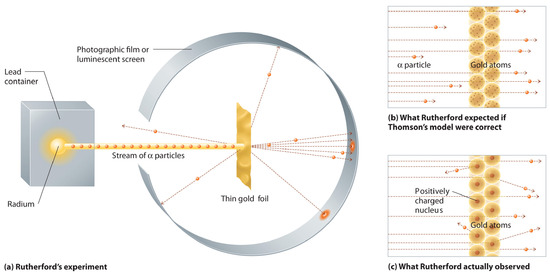
In a single famous experiment, however, Rutherford showed unambiguously that Thomson’s model of the atom was incorrect. Rutherford aimed a stream of α particles at a very thin gold foil target (part (a) in Figure 1.21) and examined how the α particles were scattered by the foil. Gold was chosen because it could be easily hammered into extremely thin sheets, minimizing the number of atoms in the target. If Thomson’s model of the atom were correct, the positively-charged α particles should crash through the uniformly distributed mass of the gold target like cannonballs through the side of a wooden house. They might be moving a little slower when they emerged, but they should pass essentially straight through the target (part (b) in Figure 1.21). To Rutherford’s amazement, a small fraction of the α particles were deflected at large angles, and some were reflected directly back at the source (part (c) in Figure 1.21). According to Rutherford, “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Figure 1.21 A Summary of Rutherford’s Experiments. (a) A representation of the apparatus Rutherford used to detect deflections in a stream of α particles aimed at a thin gold foil target. The particles were produced by a sample of radium. (b) If Thomson’s model of the atom were correct, the α particles should have passed straight through the gold foil. (c) However, a small number of α particles were deflected in various directions, including right back at the source. This could be true only if the positive charge were much more massive than the α particle. It suggested that the mass of the gold atom is concentrated in a very small region of space, which he called the nucleus.
Rutherford’s results were not consistent with a model in which the mass and positive charge are distributed uniformly throughout the volume of an atom. Instead, they strongly suggested that both the mass and positive charge are concentrated in a tiny fraction of the volume of an atom, which Rutherford called the nucleus. It made sense that a small fraction of the α particles collided with the dense, positively charged nuclei in either a glancing fashion, resulting in large deflections, or almost head-on, causing them to be reflected straight back at the source.
Although Rutherford could not explain why repulsions between the positive charges in nuclei that contained more than one positive charge did not cause the nucleus to disintegrate, he reasoned that repulsions between negatively charged electrons would cause the electrons to be uniformly distributed throughout the atom’s volume.Today it is known that strong nuclear forces, which are much stronger than electrostatic interactions, hold the protons and the neutrons together in the nucleus. For this and other insights, Rutherford was awarded the Nobel Prize in Chemistry in 1908. Unfortunately, Rutherford would have preferred to receive the Nobel Prize in Physics because he considered physics superior to chemistry. In his opinion, “All science is either physics or stamp collecting.”
Subsequently, Rutherford established that the nucleus of the hydrogen atom was a positively charged particle, for which he coined the name proton in 1920. He also suggested that the nuclei of elements other than hydrogen must contain electrically neutral particles with approximately the same mass as the proton. The neutron, however, was not discovered until 1932, when James Chadwick (1891–1974, a student of Rutherford; Nobel Prize in Physics, 1935) discovered it. As a result of Rutherford’s work, it became clear that an α particle contains two protons and neutrons, and is therefore the nucleus of a helium atom.
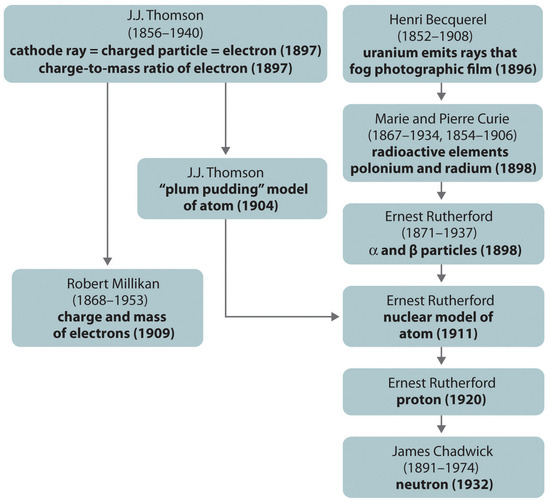
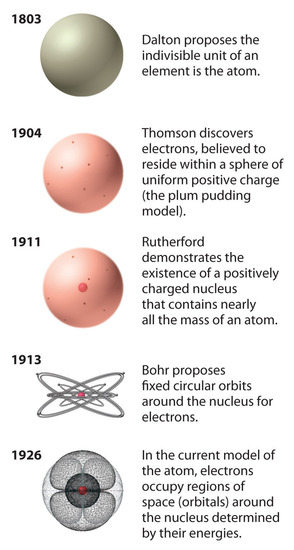
The historical development of the different models of the atom’s structure is summarized in Figure 1.22. Rutherford’s model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom’s volume. Instead, they are distributed according to a set of principles described in Chapter 6, "The Structure of Atoms." Figure 1.23 shows how the model of the atom has evolved over time from the indivisible unit of Dalton to the modern view taught today.
Figure 1.22 A Summary of the Historical Development of Models of the Components and Structure of the Atom
The dates in parentheses are the years in which the key experiments were performed.
Figure 1.23 The Evolution of Atomic Theory, as Illustrated by Models of the Oxygen Atom. Bohr’s model and the current model are described in Chapter 6, "The Structure of Atoms."
Summary
Atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons around a central nucleus composed of more massive positively charged protons and electrically neutral neutrons. Radioactivity is the emission of energetic particles and rays (radiation) by some substances. Three important kinds of radiation are α particles (helium nuclei), β particles (electrons traveling at high speed), and γ rays (similar to x-rays but higher in energy).
To date, about 115 different elements have been discovered; by definition, each is chemically unique. To understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components.
Atoms consist of electrons, protons, and neutrons. This is an oversimplification that ignores the other subatomic particles that have been discovered, but it is sufficient for discussion of chemical principles. Some properties of these subatomic particles are summarized in Table 1.3 "Properties of Subatomic Particles," which illustrates three important points:
- Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton, respectively.
- Neutrons have approximately the same mass as protons but no charge. They are electrically neutral.
- The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms.
The discovery of the electron and the proton was crucial to the development of the modern model of the atom and provides an excellent case study in the application of the scientific method. In fact, the elucidation of the atom’s structure is one of the greatest detective stories in the history of science.
Table 1.3 Properties of Subatomic Particles*
| Particle | Mass (g) | Atomic Mass (amu) | Electrical Charge (coulombs) | Relative Charge |
|---|---|---|---|---|
| * For a review of using scientific notation and units of measurement, see Essential Skills 1 (Section 1.9 "Essential Skills 1"). | ||||
| electron | \(9.109 \times 10^{-28}\) | 0.0005486 | −1.602 × 10−19 | −1 |
| proton | \(1.673 \times 10^{-24}\) | 1.007276 | +1.602 × 10−19 | +1 |
| neutron | \(1.675 \times 10^{-24}\) | 1.008665 | 0 | 0 |
KEY TAKEAWAY
The atom consists of discrete particles that govern its chemical and physical behavior.
| CONCEPTUAL PROBLEMS |
|---|
|
| NUMERICAL PROBLEMS |
|---|
| Please be sure you are familiar with the topics discussed in Essential Skills 1 (Section 1.9 "Essential Skills 1") before proceeding to the Numerical Problems.
a. electrons. b. protons.
a. electrons. b. protons.
|