IM11. Controversies in drawing structures
- Page ID
- 4075
There is sometimes controversy in science. Argument can be an important part of how we can arrive at a better understanding of things. In the context of molecules and structures, there have been long-running controversies about how to draw certain phosphorus and sulfur compounds. A look at that controversy can tell us some things about how different chemists think about bonding.
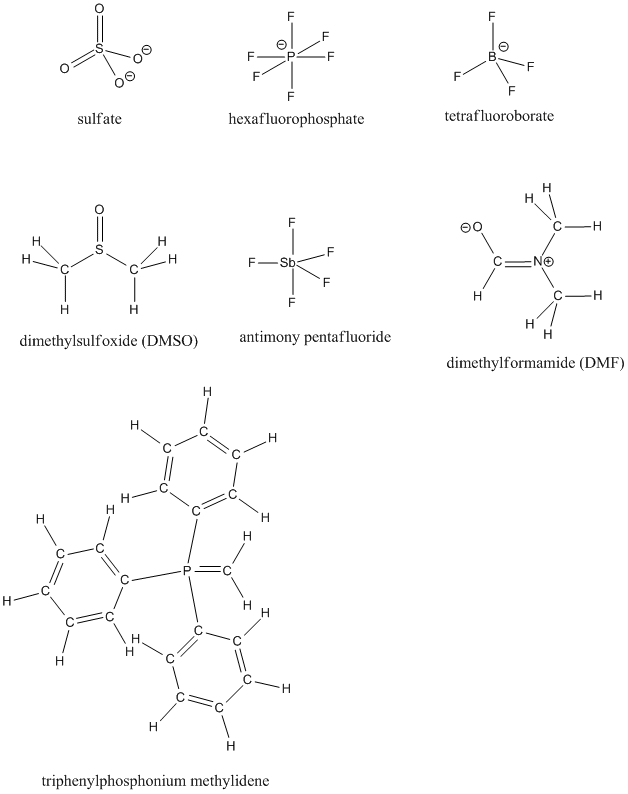
The controversy concerns how to draw, and think about, the bonding in a number of "high-valent" sulfur and phosphorus compounds, in which the sulfur or phosphorus atom is bonded to more than the usual number of neighbors. Sulfate ion, SO42-, is an example of a high-valent sulfur compound. Sulfur, like oxygen, frequently forms two bonds. In sulfate, the sulfur is attached to four different atoms. We could draw that structure in two ways.
- A structure that obeys the octet rule would have a single bond to each oxygen. That would satisfy the octet of sulfur. To fill in the right number of electrons for this group of atoms, each oxygen will have a negative formal charge. Sulfur will have a charge of plus two.
- An alternative way to draw the structure would include double bonds between sulfur and two of the oxygen atoms. That structure would have less charge separation than the other structure. However, the sulfur would have twelve electrons in its valence shell, not eight. It would exceed its octet.
Can an atom have more than eight electrons in its valence shell? Lots of them can, if they are further down the periodic table. Whether sulfur and phosphorus can is much less clear. Frequently, the fact that these are 3rd row atoms is considered significant. After all, the d electrons begin in the third row. Perhaps sulfur and phosphorus can accommodate extra electrons because they have d orbitals. The d orbitals do not have any electrons in them, yet, so maybe they can accept some from other atoms via bonds.
Many theoretical chemists (including experts in sulfur and phosphorus chemistry) are convinced that this scenario just is not possible. The d level in these atoms is just too high in energy. Other atoms can't possibly donate electrons to sulfur and phosphorus in this way.
And yet, many chemists (including experts in sulfur and phosphorus chemistry) still draw sulfate with double bonds. Why would they make such a mistake in the face of reason? Partly, it has to do with experimental evidence. Remember, "experimental" sounds wishy-washy and tentative to non-scientists, but to a chemist the term really means "reality-based". One of the reasons people draw double bonds in many sulfur and phosphorus compounds is that the bonds simply behave like double bonds. That is, they are stronger and shorter than single bonds. We might not think about those bonds in exactly the way we think about double bonds in other situations
Problem IM11.2.
Indicate whether each of the structures in IM11.1. obeys the octet rule. If not, provide a resonance structure that obeys the octet rule.
Problem IM11.3.
Many oxyphosphorus compounds are commonly drawn in a way that is inconsistent with the octet rule. Draw teo structures for each of the following compounds: one that obeys the octet rule but has higher charge separation, and one that has lower charge separation but exceeds the octet on phosphorus (note: in a few of the compounds, hydrogen is attached to phosphorus, as suggested in the condensed formula.)
a) H3PO4 or PO(OH)3 b) H3PO3 or HPO(OH)2 c) H3PO2 or H2PO(OH)
d) (CH3)3PO e) (CH3O)PO
Problem IM11.4.
Global warming is a controversial topic in society but there is not a great deal of controversy about it in the scientific community. A summary of evidence can be found at the National Oceanic and Atmospheric Administration's website.
Explain some of the evidence that global warming is occurring and is linked to human activity. Why do you think there is little debate about these ideas among scientists, but lots of debate in the US public as a whole?