SP7. Isomerism and
- Page ID
- 4153
Sometimes there is more than one way to connect a given group of atoms into a molecular structure. Given the formula C2H6O, two different isomers are possible: methyl ether and ethanol. They have the same formula but different structures. The word, "isomer", is from the Greek, meaning "same things", referring to their identical content. However, isomers often have very different physical and biological properties.
These two compounds have different physical properties, as you can see in the table below.
|
Why is that? What is the origin for the differences in physical properties between these two, similar compounds, one of which is a gas at room temperature while the other is a liquid? Both contain carbon-hydrogen and carbon-oxygen bonds. The ethanol also contains a carbon-carbon bond and an oxygen-hydrogen bond.
Certain bonds, or groups of bonds, confer specific behaviors on the compounds in which they are found. For instance, when an OH group is found in an organic compound, it can make the compound moderately acidic. It also makes the compound a little more likely to be a solid or liquid rather than a gas. Why?
Different collections of bonds that are commonly found in organic compounds are referred to as functional groups. Functional groups influence the physical properties of the compounds in which they are found. They can also influence the biological activity of a compound; that is, they can help determine whether a compound might be active as a specific type of drug, a hormone, or other types of compounds that regularly interact with organisms in nature.
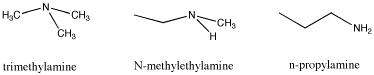
Another example of isomers is found by looking at the formula C3H9N. This time there are three different structures that can occur with the given formula. Again, the three isomers have different properties, and these differences are not just restricted to melting points and boiling points. What do you think is responsible for these differences?
|
Some of these differences may be difficult to explain. However, the differences in melting points and boiling points are quite straightforward. At least to some extent, you may be able to look at two similar structures and make an educated guess about which one will melt or evaporate more readily than the other, provided they have different functional groups that you can compare.
Problem SP7.1.
In the following pairs of isomers, which one would have the highest boiling point?
a) 2-propenol; propanal b) ethyl pentanoate; heptanoic acid c) 1-hexen-3-one; diallyl ether (or di(2-propenyl) ether)