SP5. Dipole attractions
- Page ID
- 4151
You can imagine that molecules with permanent dipoles would interact with each other much more strongly than molecules that rely on temporary dipoles in order to stick together.
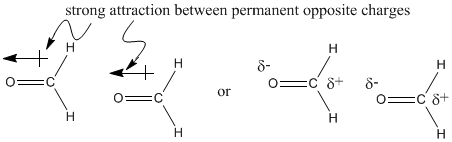
Ethane, C2H6 (sometimes written CH3CH3, suggesting two carbons are each connected to three hydrogens, and also to each other), and formaldehyde, CH2O, have different formulae but the same molecular weight. Based on weight alone, it would take about the same amount of energy to move an ethane molecule and a molecule of formaldehyde.
The two molecules also have somewhat similar shapes, unlike neopentane and pentane. At room temperature, ethane and formaldehyde are both gases. Nevertheless the two compounds have very different boiling points; formaldehyde becomes liquid around -20 oC, which would be a very cold winter day in, say, Chicago. Ethane does not become liquid unless it is cooled to around -90oC, a cold winter day on Neptune, at which point formaldehyde is just about ready to freeze solid.
The difference between these two molecules must be due to the oxygen atom in formaldehyde. Oxygen, the second most electronegative element in the periodic table, can form some very polar bonds. The permanent dipole that results between the oxygen and carbon makes the formaldehyde molecules much stickier than the ethane molecules, which depend on fleeting London interactions if they are going to hold on to each other.
There are other variations on dipole interactions that are pretty common. For example, you could imagine that a dipolar molecule would interact pretty strongly with an ion. We will take a look at that situation shortly when we think about how mixtures of different compounds interact with each other. However, perhaps the most important variation in organic chemistry is hydrogen bonding.
Problem SP5.1.
Comment on dipole moments in the following compounds and predict their relative boiling points.
a) 1-chlorobutane vs. pentane b) triethylamine vs. 3-ethylpentane c) diethyl ether vs. pentane
Problem SP5.2.
The dipole moment of a molecule (essentially the size of its dipole) can be calculated from the dielectric constant of a material, which can be measured experimentally on a bulk quantity of the compound. The dipole moment of chloromethane, 12.9 D, is higher than that of dichloromethane, 9.08 D, even though dichloromethane has a greater number of polar bonds than chloromethane. Explain why.
Problem SP5.3.
Dichloromethane nonetheless has a higher boiling point (39 °C) than chloromethane ( -24 °C). Explain why.
Problem SP5.4.
Comment on dipole moments in the following compounds and predict their relative boiling points.
- butane and ethyl methyl ether
- ethyl methyl ether and 2-propanone
- trimethylamine and propanenitrile
- 2-propanone and 2-chloropropane