13.1: Tautomers
- Page ID
- 977
You are already familiar with several types of isomeric relationships among organic molecules; constitutional isomers, conformational isomers, enantiomers, and diastereomers. Here is one more. Tautomers are two molecules with the same molecular formula but different connectivity - constitutional isomers, in other words - which can interconvert in a rapid equilibrium. The most common tautomeric relationship in organic chemistry is the keto-enol pair.
13.1A: Keto-enol tautomerization
When we draw a ketone or aldehyde using the Lewis structure convention, we show a double bond between the carbonyl carbon and the oxygen - this is known as the keto form. It turns out that ketones and aldehydes often exist in rapid equilibrium with a tautomeric form known as an enol.
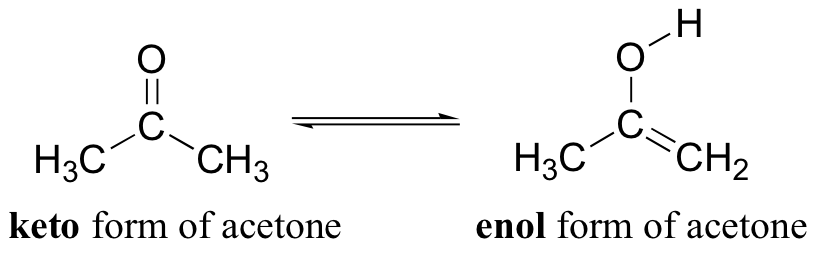
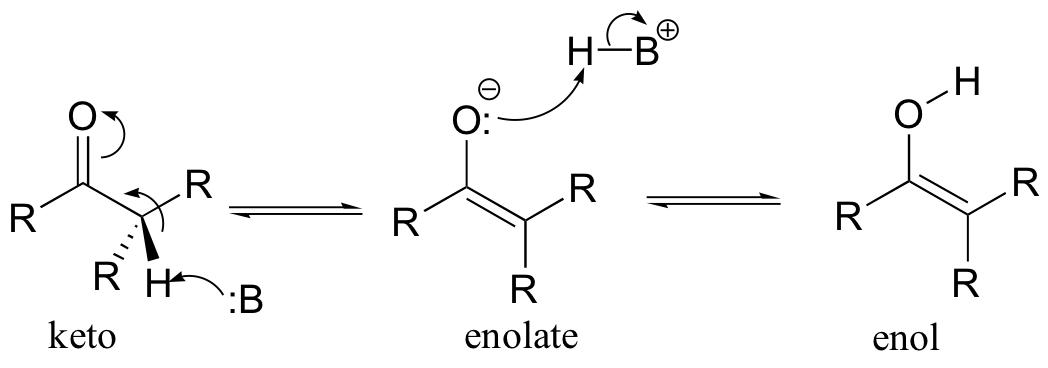
The alpha-protons of carbonyls, as you recall, are somewhat acidic: the pKa of acetone, for example, is approximately 19. In the formation of an enol, a base abstracts an a-proton from a carbonyl compound, and that same proton (or a proton on a nearby acid group) is transferred to the carbonyl oxygen. The result is a new functional group that combines both alkene and alcohol characteristics - hence the term enol. The deprotonated form of an enol is an enolate.
As a general rule, the keto form of a carbonyl is lower in energy, and thus predominates at equilibrium. Acetone, for example, is present at >99% keto form at equilibrium.
Exercise 13.1: Draw all of the possible enol forms of the following aldehydes/ketones. There may be more than one possible enol form.
- 3-pentanone
- acetaldehyde (IUPAC name ethaldehyde)
- cyclohexanone
- 2-pentanone
Exercise 13.2: Draw three examples of aldehyde or ketone compounds for which there is no possible enol form.
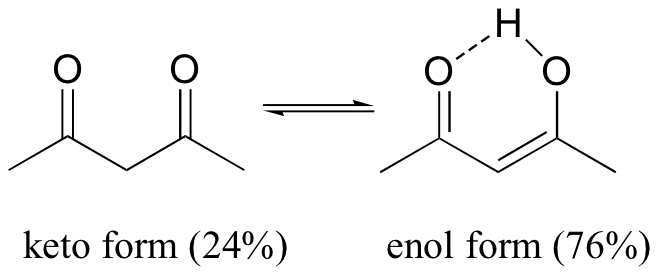
There are a few special cases where the enol form predominates at equilibrium: 2,4-pentane dione exists mostly in its enol form (76%), due to the extra stability of the conjugated double bonds that are present in the enol form, and due also to a favorable hydrogen bonding interaction.
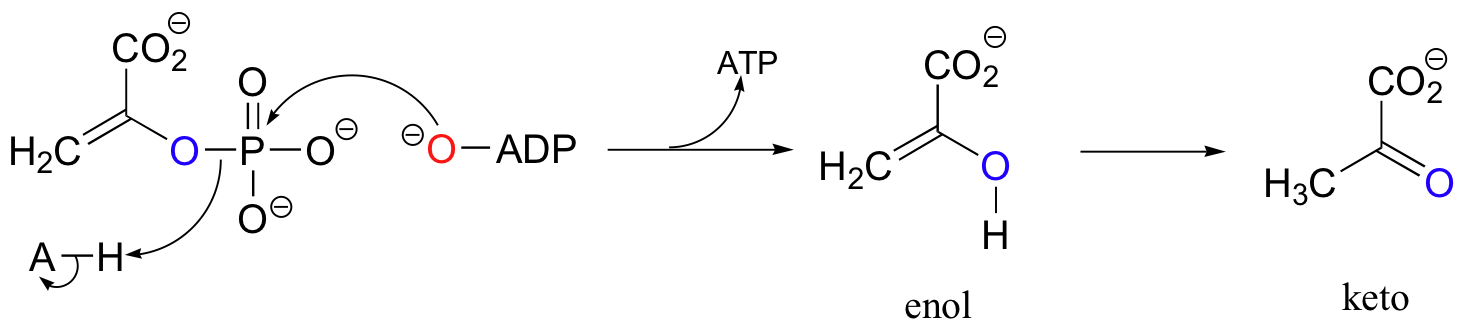
We saw a good example of an enol to keto tautomerization in the last step of the pyruvate kinase reaction (section 10.2F). The enol served as a leaving group in this phosphoryl transfer reaction, after which tautomerization occurred to form the ketone group of pyruvate.
13.1B: Imine-enamine tautomerization
Another common tautomeric relationship in biological organic chemistry is the equilibrium between imines (also known as Schiff bases) and enamines, which are the nitrogen equivalents of enols.
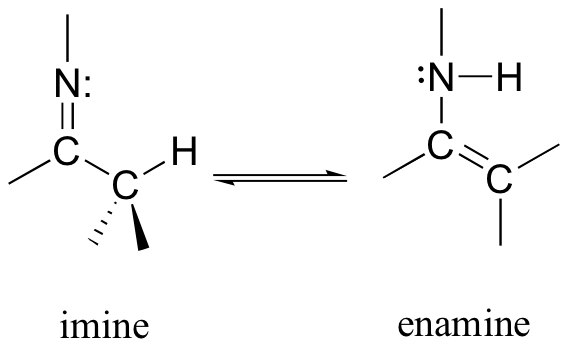
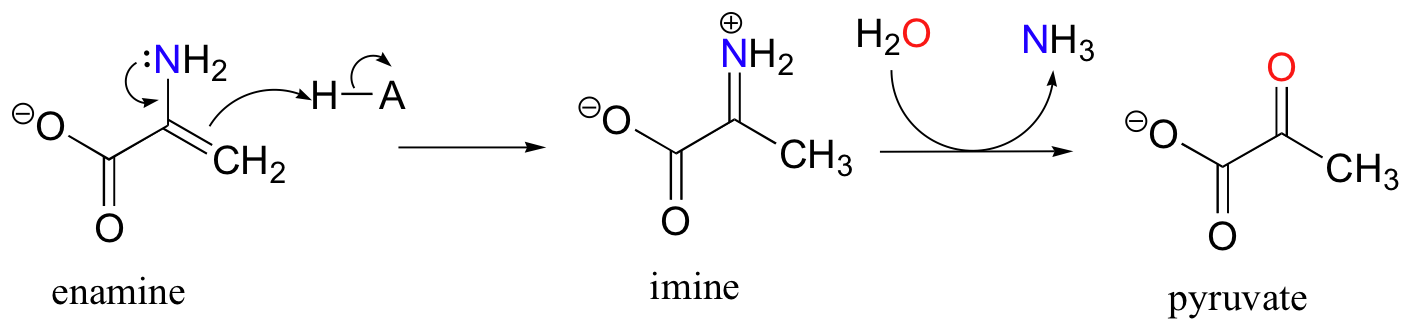
The degradation of serine, for example, involves an enamine to imine tautomerization step, followed by hydrolysis of the imine (section 11.6) to form pyruvate.
As we shall see in a later section, enamines are key intermediates in a very important type of carbon-carbon bond-forming reaction.


