7.3: Malleability of Metals and Alloys
- Page ID
- 34789
Metals with close-packed structures (hcp and ccp) such as copper, gold, silver, zinc, magnesium, etc. are in general more malleable than those with the bcc structure (tungsten, vanadium, chromium, etc.). Why? In the close-packed structure, there is relatively little corrugation between sheets of metal atoms. This means that these planes can slip past each other relatively easily. In the bcc structure, there are no close-packed planes, and much greater corrugation between atoms at different levels. This makes it much harder for one row to slide past another.
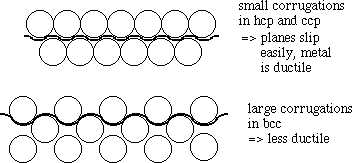
Figure: Small vs. Large corrugations in metals
This effect explains the hardness of alloys like brass (CuZn, which has a bcc structure), which are made by combining two soft metals (Cu and Zn, which are respectively ccp and hcp as pure metals, are both soft and ductile). Bronzes - originally made as alloys of copper and arsenic, but later as alloys of copper and tin - are harder than either of the constituent metals for the same reason. In the Bronze Age, possession of these harder alloys provided a tactical advantage in warfare (see image below), that was later supplanted when the technology for smelting iron was developed.

Figure: Bronze age weapons from Romania
Contributors
Adapted from the Wikibook constructed by Chemistry 310 students at Penn State University.

