4.5: Pourbaix diagrams
- Page ID
- 34767
Pourbaix Diagrams plot electrochemical stability for different redox states of an element as a function of pH. As noted above, these diagrams are essentially phase diagrams that plot the map the conditions of potential and pH (most typically in aqueous solutions) where different redox species are stable. We saw a simple example of such a diagram in section 4.5 for H2O. Typically, the water redox reactions are plotted as dotted lines on these more complicated diagrams for other elements.
The lines in Pourbaix diagrams represent redox and acid-base reactions, and are the parts of the diagram where two species can exist in equilibrium. For example, in the Pourbaix diagram for Fe below, the horizontal line between the Fe3+ and Fe2+ regions represents the reaction \(Fe^{3+}_{(aq)} + e^- \rightleftharpoons Fe^{2+}_{(aq)}\), which has a standard potential of +0.77 V. While we could use standard potentials for all these lines, but in practice Pourbaix diagrams are usually plotted for lower ion concentrations (often 1 mM) that are more relevant to corrosion and electrochemical experiments.
Iron Pourbaix diagram
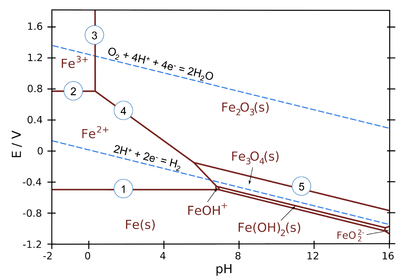
Figure 4.5.1: Pourbaix diagram for iron at ionic concentrations of 1.0 mM
- Areas in the Pourbaix diagram mark regions where a single species (Fe2+(aq), Fe3O4(s), etc.) is stable. More stable species tend to occupy larger areas.
- Lines mark places where two species exist in equilibrium.
- Pure redox reactions are horizontal lines - these reactions are not pH-dependent
- Pure acid-base reactions are vertical lines - these do not depend on potential
- Reactions that are both acid-base and redox have a slope of -0.0592 V/pH x # H+⁄# e-)
Examples of equilibria in the iron Pourbaix diagram (numbered on the plot):
- \(Fe^{2+} + 2e^- \rightarrow Fe(s)\) (pure redox reaction - no pH dependence)
- \(Fe^{3+} + e^- \rightarrow Fe^{2+}\) (pure redox reaction - no pH dependence)
- \(2 Fe^{3+} + 3 H_2O \rightarrow Fe_2O_3(s)+ 6H^+\) (pure acid-base, no redox)
- \(2 Fe^{2+} + 3 H_2O \rightarrow Fe_2O_3(s)+ 6H^+ + 2e^-\) (slope = -59.2 x 6/2 = -178 mV/pH)
- \(2 Fe_3O_4(s) + H_2O \rightarrow 3 Fe_2O_3(s) + 2H^+ + 2e^-\) (slope = -59.2 x 2/2 = -59.2 mV/pH)
The water redox lines have special significance on a Pourbaix diagram for an element such as iron. Recall that liquid water is stable only in the region between the dotted lines. Below the H2 line, water is unstable relative to hydrogen gas, and above the O2 line, water is unstable with respect to oxygen. For active metals such as Fe, the region where the pure element is stable is typically below the H2 line. This means that iron metal is unstable in contact with water, undergoing reactions:
\[\ce{Fe(s) + 2H^+ \rightarrow Fe^{2+}(aq) + H_2 } \ (in \ acid)\]
\[\ce{Fe(s) + 2 H_2O \rightarrow Fe(OH)_2(s) + H_2 } \ (in \ base)\]
Iron (and most other metals) are also thermodynamically unstable in air-saturated water, where the potential of the solution is close to the O2 line in the Pourbaix diagram. Here the spontaneous reactions are:
\[\ce{4 Fe(s) + 3 O_2 + 12H^+ \rightarrow 4 Fe^{3+} + 6 H_2O} \ (in \ acid)\]
\[\ce{4 Fe(s) + 3 O_2 \rightarrow 2 Fe_2O_3(s)} \ (in \ base)\]
Corrosion and Passivation
It certainly sounds bad for our friend Fe: unstable in water, no matter what the pH or potential. Given enough time, it will all turn into rust. But iron (and other active metals) can corrode, or can be stabilized against corrosion, depending on the conditions. Because our civilization is dependent on the use of active metals such as Fe, Al, Zn, Ti, Cr... for practically everything, it is important to understand this, and we can do so by referring to the Pourbaix diagram.
The corrosion of iron (and other active metals such as Al) is indeed rapid in parts of the Pourbaix diagram where the element is oxidized to a soluble, ionic product such as Fe3+(aq) or Al3+(aq). However, solids such as Fe2O3, and especially Al2O3, form a protective coating on the metal that greatly impedes the corrosion reaction. This phenomenon is called passivation.
Draw a vertical line through the iron Pourbaix diagram at the pH of tap water (about 6) and you will discover something interesting: at slightly acidic pH, iron is quite unstable with respect to corrosion by the reaction:
\[Fe_{(s)} + 2H^+ \rightarrow Fe^{2+}_{(aq)} + H_2\]
but only in water that contains relatively little oxygen, i.e., in solutions where the potential is near the H2 line. Saturating the water with air or oxygen moves the system closer to the O2 line, where the most stable species is Fe2O3 and the corrosion reaction is:
\[4 Fe_{(s)} + 3 O_2 \rightarrow 2 Fe_2O_3(s)\]
This oxidation reaction is orders of magnitude slower because the oxide that is formed passivates the surface. Therefore iron corrodes much more slowly in oxygenated solutions. More generally, iron (and other active metals) are passivated whenever they oxidize to produce a solid product, and corrode whenever the product is ionic and soluble. This behavior can be summed up on the color-coded Pourbaix diagram below:
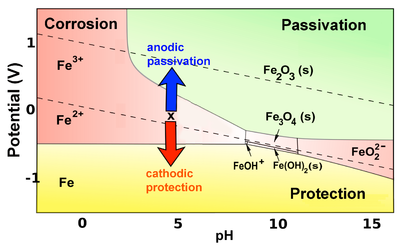
In the yellow part of the diagram, an active metal such as iron can be protected by a second mechanism, which is to bias it so that its potential is below the oxidation potential of the metal. This cathodic protection strategy is most frequently carried out by connecting a more active metal such as Mg or Zn to the iron or steel object (e.g., the hull of a ship, or an underground gas pipeline) that is being protected. The active metal (which must be higher than Fe in the activity series) is also in contact with the solution and slowly corrodes, so it must eventually be replaced. In some cases a battery - the anode of which oxidizes water to oxygen in the solution - is used instead to apply a negative bias.
Another common mode of corrosion of iron and carbon steel is differential aeration. In this case, part of the iron object - e.g., the base of a bridge, or the drill in an oil rig - is under water or in an anoxic environment such as mud or soil. The potential of the solution is close to the H2 line in the Pourbaix diagram, where Fe can corrode to Fe2+ (aq). Another part of the iron object is in the air, or near the surface where water is well oxygenated. At that surface oxygen can be reduced to water, O2 + 4H+ + 4e- = 2 H2O. The conductive iron object completes the circuit, carrying electrons from the anode (where Fe is oxidized) to the cathode (where O2 is reduced). Corrosion by differential aeration can be rapid because soluble ions are produced, and the reaction has a driving force of over 1 V. Iron or carbon steel that is subjected to frequent weathering, such as the cast iron bridge and lampost shown at the right, is corroded on the surface by differential aeration.
Differential aeration is involved in the formation of a rust ring around wet areas of cast iron, e.g., an iron frying pan left partially submerged in water for a day or more. (You may have seen this mechanism of corrosion in action when you did not get to the dirty dishes right away). Under the water, Fe is oxidized to soluble Fe2+, and at the water line O2 is reduced to H2O. As Fe2+ ions diffuse towards the water surface, they encounter oxygen molecules and are oxidized to Fe3+. However Fe3+ is insoluble at neutral pH and deposits as rust, typically just below the water line, forming the rust ring.
Contributors
Adapted from the Wikibook constructed by Chemistry 310 students at Penn State University.

