Answers to Chapter 12 Study Questions
- Page ID
- 11188
-
- 1 mole \(\ce{CO2}\)
- \(\mathrm{0.050 - 0.030\: moles/liter = 0.020\: moles/liter}\)
- \(\mathrm{conc.\: of\: CO_2\: produced = conc.\: of\: CH_4\: used\: up}\)
\(\mathrm{conc.\: of\: CO_2\: after\: 20\: min\: = 0.050 - 0.020 = 0.030\: mol/L}\)
- \(\mathrm{rate=\dfrac{\Delta [CO_2]}{\Delta time}}\)
- \(\mathrm{rate=\dfrac{\Delta[CO_2]}{\Delta time}=\dfrac{0.030-0.020\,mol/L}{20-10\,min}=\dfrac{0.0010\,mol/L}{min}}\)
- \(\mathrm{conc.\: of\: CO_2\: after\: 30\: min = 0.050 - 0.015 = 0.035\: mol/L}\)
\(\mathrm{rate=\dfrac{\Delta[CO_2]}{\Delta time}=\dfrac{0.035-0\,mol/L}{30-0\,min}=\dfrac{1.2\times10^{-3}\,mol/L}{min}}\)
- \(\mathrm{rate=\dfrac{\Delta[CO_2]}{\Delta time}=-\dfrac{\Delta [O_2]}{2\Delta t}}\); \(\mathrm{\dfrac{\Delta [O_2]}{\Delta t}=-2\dfrac{\Delta[CO_2]}{\Delta time}}\)
- \(\mathrm{rate=\dfrac{\Delta [O_2]}{\Delta t}=-2\dfrac{\Delta[CO_2]}{\Delta time}}\) from e) \(\mathrm{\dfrac{\Delta [CO_2]}{\Delta time}=\dfrac{0.0010 \, mol/L}{min}}\)
\(\mathrm{\dfrac{\Delta [O_2]}{\Delta t} = -2(0.0010) = \dfrac{- 0.0020\: mol/L}{min}}\)
- 1 mole \(\ce{CO2}\)
-
- The mechanism, and therefore the number of steps in a reaction, are determined experimentally.
- The second step; it's the slowest step.
- \(\mathrm{rate = k \times (concentration\: NO_3) \times (concentration\: NO)}\)
- rate increases by a factor of \(\mathrm{\dfrac{3}{2}}\)
\(\mathrm{\left(\dfrac{1}{2} \times 3 = \dfrac{3}{2}\right)}\) - the order with respect to \(\ce{NO}\) is 1; the overall order is 2.
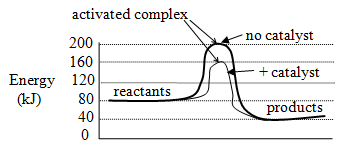
-
- concentration of reactants: Reaction rate increases as concentration of reactants increases because number of collisions increases, making reaction more likely to occur.
- surface area of reactants: Rate increases as surface area of reactants increases because the greater the area of reactant exposed, the more likely are collisions that will result in product formation.
- temperature: As temperature increases, rate increases because at higher temperature, a greater proportion of reactant molecules have a kinetic energy greater than the activation energy so a greater proportion of collisions result in product formation.
- catalyst: Catalysts increase reaction rate by lowering the activation energy.
- inhibitors: Inhibitors decrease reaction rate by destroying a catalyst, reducing effective surface area or by using up reactant.
-
- \(\ce{2 N2O(g) \rightarrow 2 N2(g) + O2(g)}\)
- \(\ce{N2O(g) \rightarrow N2 + O}\); \(\ce{N2O(g) + O \rightarrow N2 + O2}\)
- \(\ce{O}\)
-
- \(\mathrm{rate = k [HgCl_2]^n \times [C_2O_4^{2-}]^m}\)
\(\mathrm{\dfrac{rate_2}{rate_1}=\dfrac{k[HgCl_2]_2^n}{k[HgCl_2]_1^n}=\left(\dfrac{[HgCl_2]_2}{[HgCl_2]_1}\right)^n}\)
\(\mathrm{\dfrac{2.6\times10^{-7}}{1.3\times10^{-7}}=\left(\dfrac{0.20}{0.10}\right)^n}\)
\(\mathrm{2 = 2^n}\) ; \(\mathrm{n = 1}\)
order with respect to \(\ce{HgCl2}\) is 1. (Rate is proportional to \(\ce{[HgCl2]}\).)
\(\mathrm{\dfrac{rate_2}{rate_1}=\dfrac{k[C_2O_4^{2-}]_2^m}{k[C_2O_4^{2-}]_1^m}=\left(\dfrac{[C_2O_4^{2-}]_2}{[C_2O_4^{2-}]_1}\right)^m}\)
\(\mathrm{\dfrac{5.2\times10^{-7}}{1.3\times10^{-7}}=\left(\dfrac{0.20}{0.10}\right)^m}\)
\(\mathrm{4 = 2^m}\) ; \(\mathrm{m = 2}\)
order with respect to \(\ce{C_2O_4^{2-}}\) is 2. Overall order is 3.
- \(\mathrm{rate = k [HgCl_2] [C_2O_4^{2-}]^2}\)
- \(\mathrm{rate = k [HgCl_2] [C_2O_4^{2-}]^2}\) ; \(\mathrm{1.3 \times 10^{-7} = k (0.10)(0.10)^2}\); \(\mathrm{k = \dfrac{1.3 \times 10^{-7}}{0.001} = 1.3 \times 10^{-4}}\)
- \(\mathrm{rate = 1.3 \times 10^{-4} \times [HgCl_2] [C_2O_4^{2-}]^2}\); \(\mathrm{rate = 1.3 \times 10^{-4} \times (0.30) (0.30)^2 = \dfrac{3.5 \times 10^{-6}\:mol/L}{s}}\)

