Conservation of Energy
- Page ID
- 1921
The law of Conservation of Energy refers to an isolated system in which there is no net change in energy and where energy is neither created nor destroyed. Although there is no change in energy, energy can change forms, for example from potential to kinetic energy. In other words, potential energy (V) and kinetic energy (T) sum to a constant total energy (E) for a specific isolated system.
\(E = T + V\)
Another way that energy can change forms is heat (q) and work (w). As heat is applied to a closed system, the system does work by increasing its volume.
\(w = P_{ext}\Delta{V}\)
where Pext is the external pressure, and delta V is the change in volume. A classic example of this is a piston. As heat is added to the cylinder, the pressure inside the cylinder increases. The piston then rises to relieve the pressure difference between the pressure inside the cylinder and the external pressure. By increasing the volume in the cylinder, the piston has just done work. Reference the picture below.
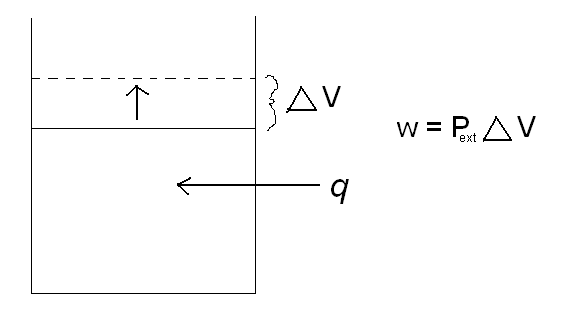
The sum of heat and work is the change in internal energy, \(\Delta{U}\).
In an isolated system, \(q = -w\). Therefore, \(\Delta{U} = 0\).
In quantum mechanics, the equation
\(\hat{H}\psi_n = E_n\psi_n\)
Where,
- \(E\) = the energy corresponding to a wave function
- \(V\) = the potential
- \(\hat{H}\) = the Hamiltonian operator
The equation is analogous to the equation:
\(E = T + V\)
Contributors and Attributions
- Vanessa Chan

