Non-Ideal Mixtures of Liquids
- Page ID
- 3870
This page looks at the phase diagrams for non-ideal mixtures of liquids, and introduces the idea of an azeotropic mixture (also known as an azeotrope or constant boiling mixture). It goes on to explain how this complicates the process of fractionally distilling such a mixture.
Ideal Solutions follow Raoult's Law and Real Solutions Do Not
Due to Raoult's Law, a plot the vapor pressure of an ideal mixture of two liquids against their composition will result in a straight line:
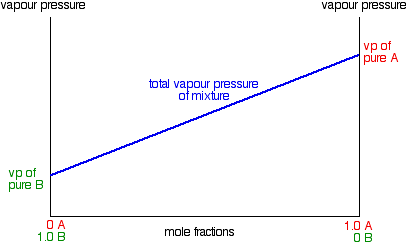
In this case, pure A has the higher vapor pressure and so is the more volatile component. Raoult's Law only works for ideal mixtures. In these, the forces between the particles in the mixture are exactly the same as those in the pure liquids. The tendency for the particles to escape is the same in the mixture and in the pure liquids. That's not true in non-ideal mixtures.
In mixtures showing a positive deviation from Raoult's Law, the vapor pressure of the mixture is always higher than you would expect from an ideal mixture. The deviation can be small - in which case, the straight line in the last graph turns into a slight curve.
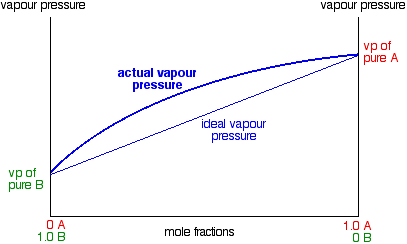
Notice that the highest vapor pressure anywhere is still the vapor pressure of pure A. Cases like this, where the deviation is small, behave just like ideal mixtures as far as distillation is concerned, and we do not need to say anything more about them. But some liquid mixtures have very large positive deviations from Raoult's Law, and in these cases, the curve becomes very distorted.
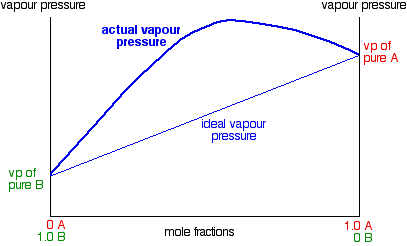
Notice that mixtures over a range of compositions have higher vapor pressures than either pure liquid. The maximum vapor pressure is no longer that of one of the pure liquids. This has important consequences when we look at boiling points and distillation further down the page. The fact that the vapor pressure is higher than ideal in these mixtures means that molecules are breaking away more easily than they do in the pure liquids. That is because the intermolecular forces between molecules of A and B are less than they are in the pure liquids.
You can see this when you mix the liquids. Less heat is evolved when the new attractions are set up than was absorbed to break the original ones. Heat will therefore be absorbed when the liquids mix. The enthalpy change of mixing is endothermic. The classic example of a mixture of this kind is ethanol and water. This produces a highly distorted curve with a maximum vapor pressure for a mixture containing 95.6% of ethanol by mass.
In exactly the same way, you can have mixtures with vapor pressures which are less than would be expected by Raoult's Law. In some cases, the deviations are small, but in others they are much greater giving a minimum value for vapor pressure lower than that of either pure component.
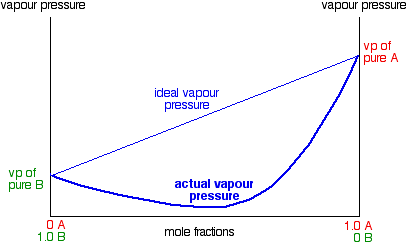
These are cases where the molecules break away from the mixture less easily than they do from the pure liquids. New stronger forces must exist in the mixture than in the original liquids. You can recognize this happening because heat is evolved when you mix the liquids - more heat is given out when the new stronger bonds are made than was used in breaking the original weaker ones. Many (although not all) examples of this involve actual reaction between the two liquids. The example of a major negative deviation is a mixture of nitric acid and water. These two covalent molecules react to give hydroxonium ions and nitrate ions.
\[ \ce{H2O(l) + HNO3(l) <=> H3O^{+} (aq) + NO3(aq)^{-}} \label{1}\]
You now have strong ionic attractions involved which increases the intermolecular interactions.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


