Early Atomic Theory
- Page ID
- 22758
The concept of the atom (Greek: atomos, "indivisible"), an indivisible particle of matter, goes back to ancient Greece and a man named Democritus, a rival of Aristotle. Democritus held that all matter could be subdivided only until some finite particle was reached. Aristotle had a quite different idea, that matter was a continuous substance, not composed of any fundamental units. And Aristotle was much more famous and admired in his time and so his error persisted into the late 1700's. But eventually work by men such as Lavoisier began to suggest that Aristotle had been seriously wrong.
Dalton's Contributions
In 1808 the first statement of a modern atomic theory was published by John Dalton, a Quaker schoolmaster from Manchester. It may not seem like much, but such a theory was used to explain two of the major laws in chemistry: the Law of Conservation of Mass and the Law of Constant (definite) Composition. And the third postulate led Dalton to formulate the Law of Multiple Proportions.
John Dalton apparently thought that atoms were pretty much little solid spheres without internal structure. His experiments with gases eventually led him to the idea that matter came in small indivisible units but his use of the word "atom" was variable. He described what we now call molecules as "atoms" and was convinced that the simplest binary compound of any two elements would have those atoms in a 1:1 ratio. According to Dalton, water would be HO. This led to a relative mass scale for atoms that was mostly in error.
Dalton also had his own notation for some of the elements he recognized.
Dalton's "modern" atomic theory, in todays terms, would be something like this:
- all matter consists of indivisible atoms which cannot be created or destroyed
- atoms of one element cannot be converted into atoms of another element
- atoms of an element are identical to one another but different from atoms of other elements
- compounds result from combinations of atoms in specific ratios
Some contemporaries of Dalton remained unimpressed. An eminent organic chemist of the time, Adolf Kolbe, said in 1877, "Dalton's atoms are no more than stupid hallucinations...mere table-tapping and supernatural explanations."
Experimental Evidence
The first evidence for sub-atomic particles came from experiments with the conduction of electricity through gases in sealed glass tubes at low pressures.

Figure : J.J. Thomson (seen here in his lab with one of his many hand-made cathode ray tubes)
Associated with the flow of electricity in such a tube are rays which originate from the negative electrode--the cathode. Thus these tubes have been called cathode ray tubes. From careful experiments with cathode ray tubes J.J. Thomson demonstrated in 1897 that the rays consist of a stream of negatively charged particles which he called electrons. He was able to measure the charge/mass ratio of these particles and found this to be the same regardless of what gas was in the tube or what metal the electrodes were made from.
In 1909 Robert Millikan used the classic oil drop experiment to determine the charge on these particles. Using the smallest charge obtained and Thomson's charge/mass ratio the electron mass is roughly 1/2000 the mass of the lightest atom. Thus there are obviously particles smaller than atoms.
Millikan used his apparatus to make many measurements of the effect of the electric field vs. gravity on the charged oil droplets. His eventual conclusion was that the charges on the drops were either equal to or multiples of one number which he decided was the charge of a single electron. With this value, and the charge/mass ratio that Thomson had measured earlier it was possible to calculate the mass of the electron.
Internal Structure
Thomson's dilemma: how could matter containing electrons be neutral and where was all the mass? Other experiments with discharge tubes suggested the existence of a positive particle with much greater mass (the proton). Based on this evidence Thomson proposed the first atomic model with sub-atomic particles.
Thomson's work (and the work of others) with cathode ray tubes eventually identified a much more massive positive particle apparently common to all matter. These were called protons. With the ingredients needed to keep matter neutral, Thomson speculated and calculated just how it might all hold together. His conclusion has become known as the "plum pudding" model of the atom and was the first to include sub-atomic particles with charges.
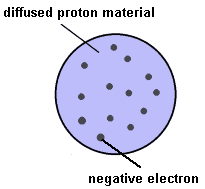
Thomson's calculations indicated that to remain stable atoms might need to have hundreds, if not thousands, of electrons. However, the developing techniques for measuring relative atomic masses seemed to contradict this, suggesting instead that less than 100 electrons would be more likely.
Finally in the years between 1908 and 1911 Ernest Rutherford and his students Hans Geiger and Ernest Marsden performed the famous gold-foil experiment in which the nuclear arrangement of the atom was discovered. Rutherford's vision of the atom as a kind of "solar system" in miniature is what most people probably carry around in their heads. As appealingly simple as it is, Rutherford's model contained a serious flaw.
Rutherford's model of the atom was no doubt appealing in part because it seemed to mimic the larger world people could see. But just as the planets slowly lose orbital energy as they circle the sun, the energy supply of the tiny electron should be degraded too.
Physicists had already established in Rutherford's era that a charged particle moving in a circle, but keeping a constant orbital radius must radiate energy. Hence the nuclear model of the atom should render all matter unstable as electrons gradually spiral into nuclei when their orbits "decay" due to loss of energy. Obviously, this does not happen.
The next step in the development of the atomic model changed the way in which scientists looked at and thought about matter, especially very small pieces like electrons and protons.

