6.3: Quantum-Mechanical Description of the Harmonic Oscillator
- Page ID
- 4508
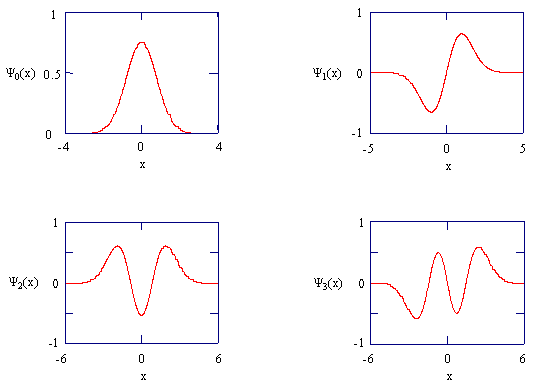
Figure \(\PageIndex{3}\).6: The harmonic oscillator wavefunctions describing the four lowest energy states.
In completing Exercise \(\PageIndex{23}\), you should have noticed that as the quantum number increases and becomes very large, the probability distribution approaches that of a classical oscillator. This observation is very general. It was first noticed by Bohr, and is called the Bohr Correspondence Principle. This principle states that classical behavior is approached in the limit of large values for a quantum number. A classical oscillator is most likely to be found in the region of space where its velocity is the smallest. This situation is similar to walking through one room and running through another. In which room do you spend more time? Where is it more likely that you will be found?


