Chapter 13 Solutions
- Page ID
- 1111
In-chapter exercises
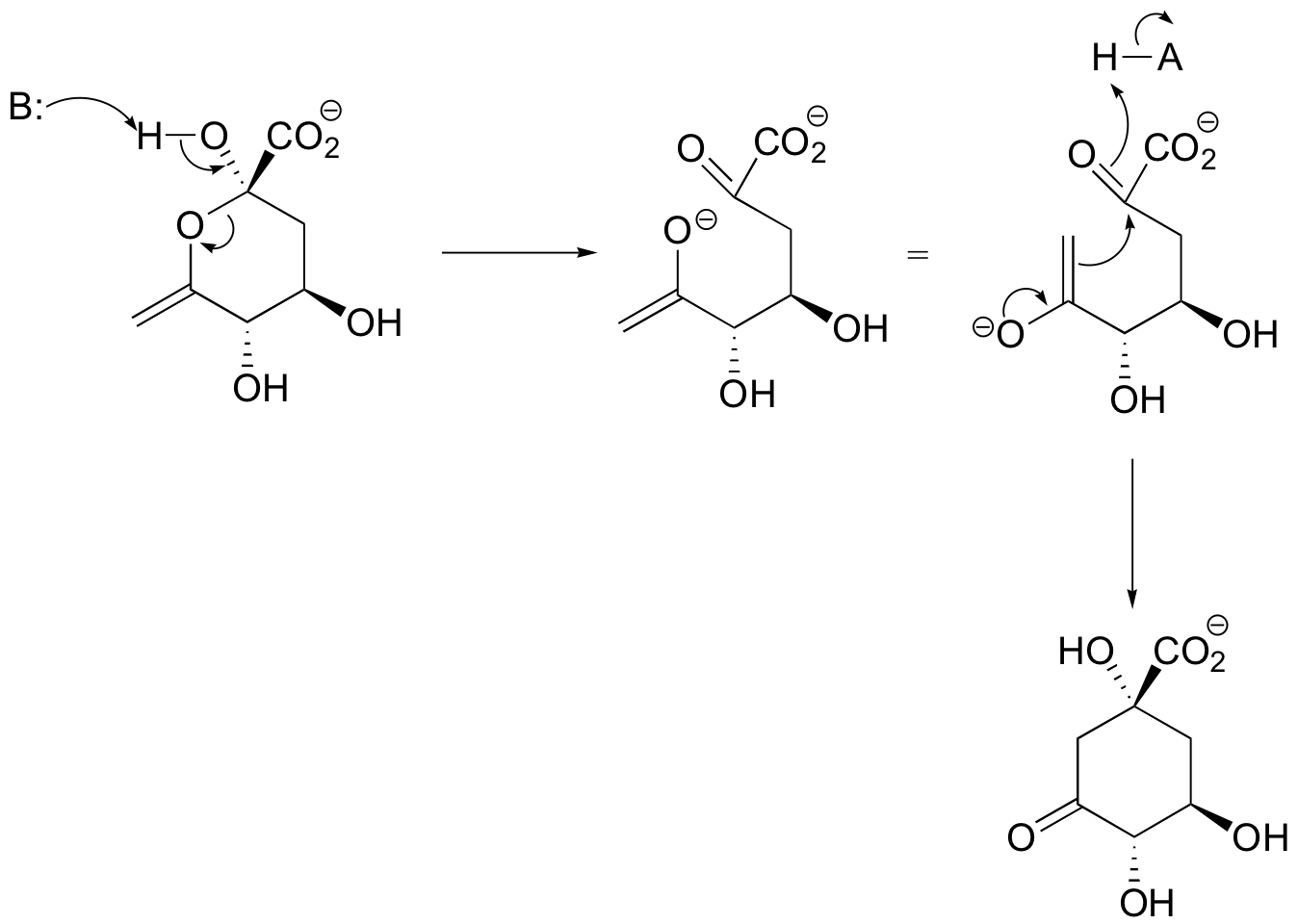
E13.1:
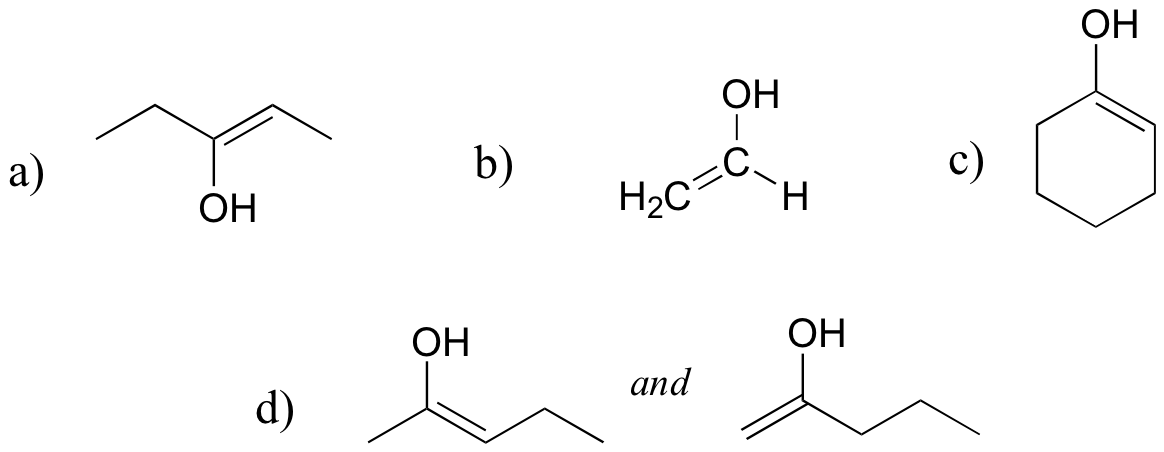
E13.2:
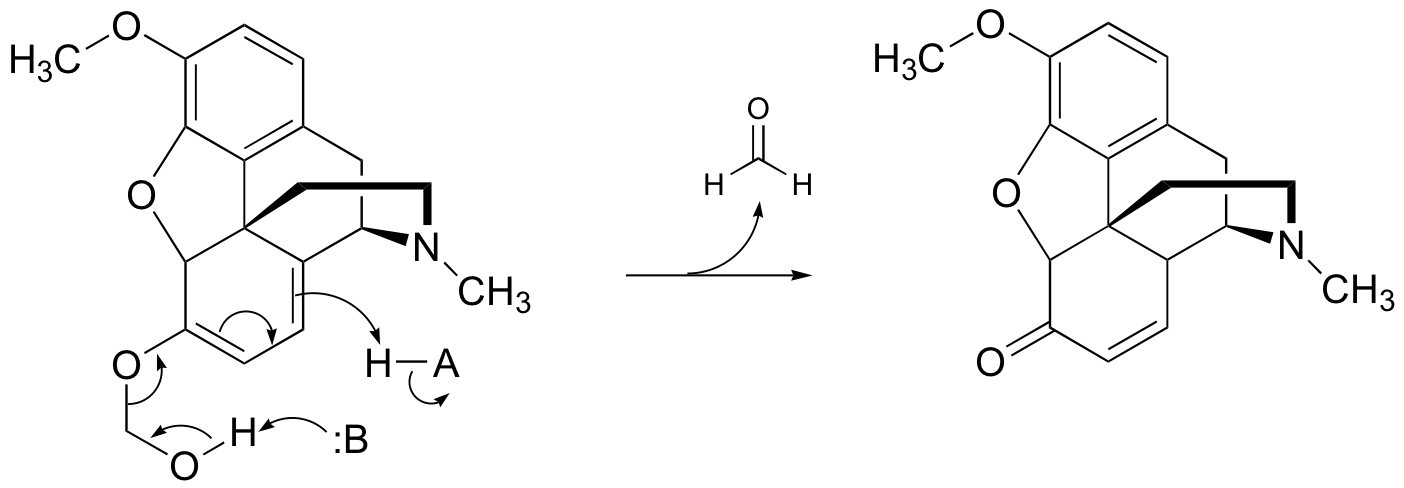
There are many possibilities: if there is no alpha-proton present, there is no enol form possible. Here are three such compounds:
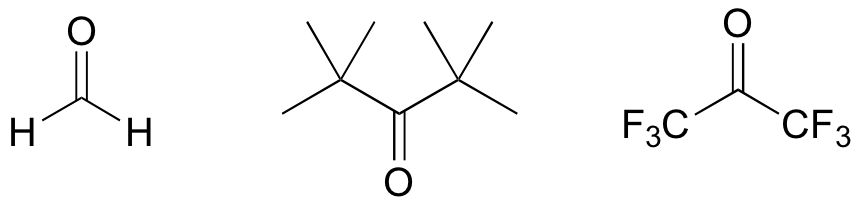
E13.3:
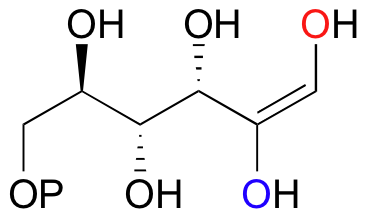
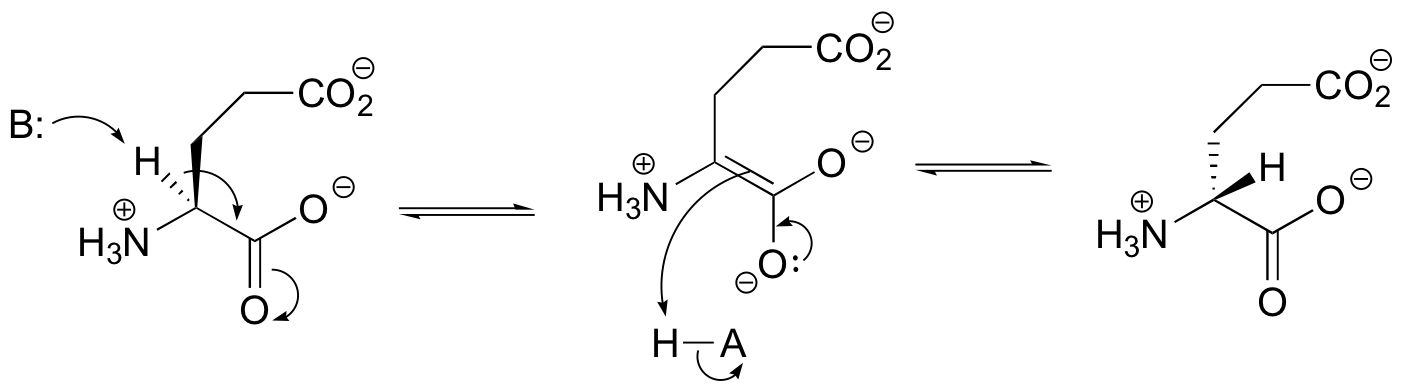
E13.4: Propose a likely mechanism for glutamate racemase, showing stereochemistry throughout.
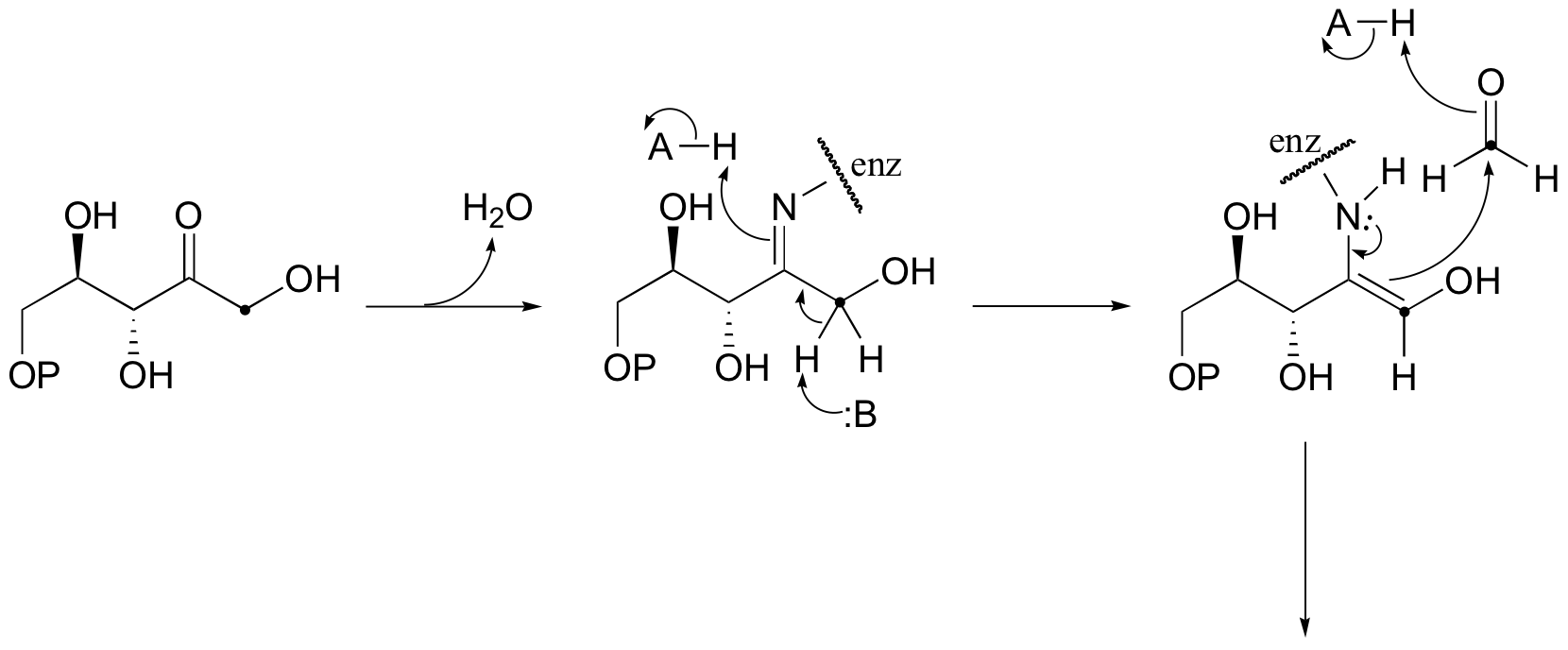
E13.5:
a)
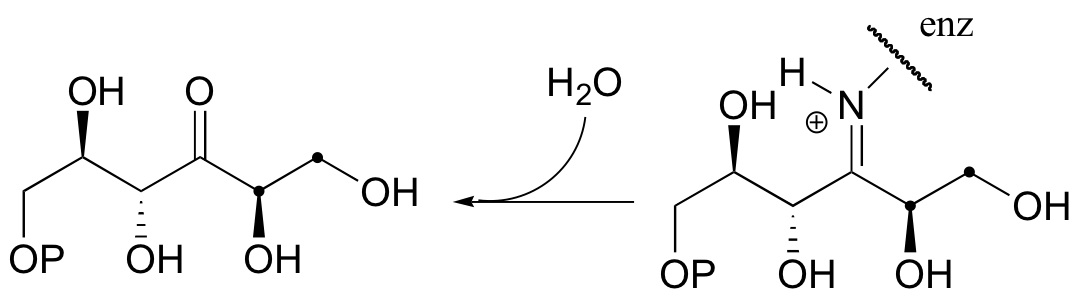
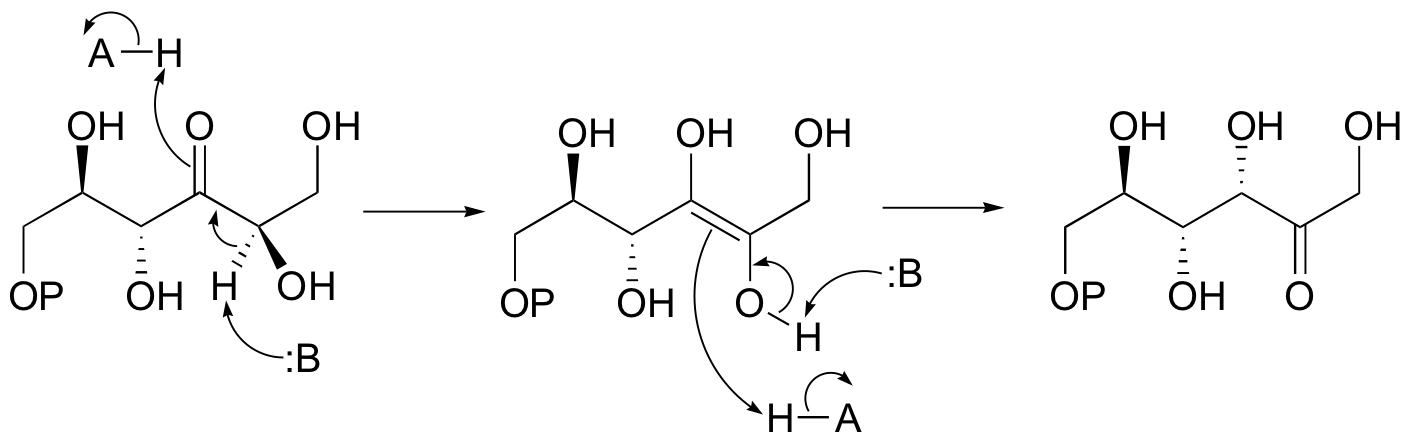
b)
E13.6:
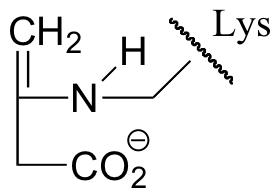
E13.7:
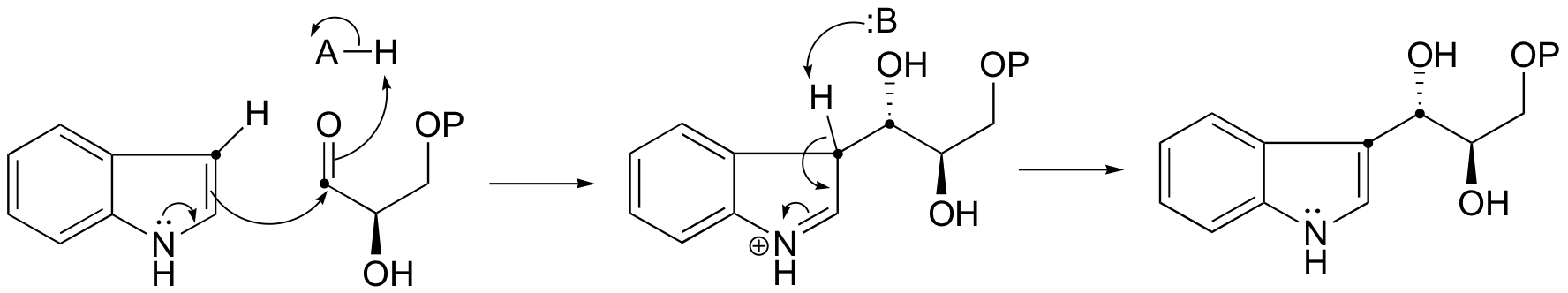
E13.8:
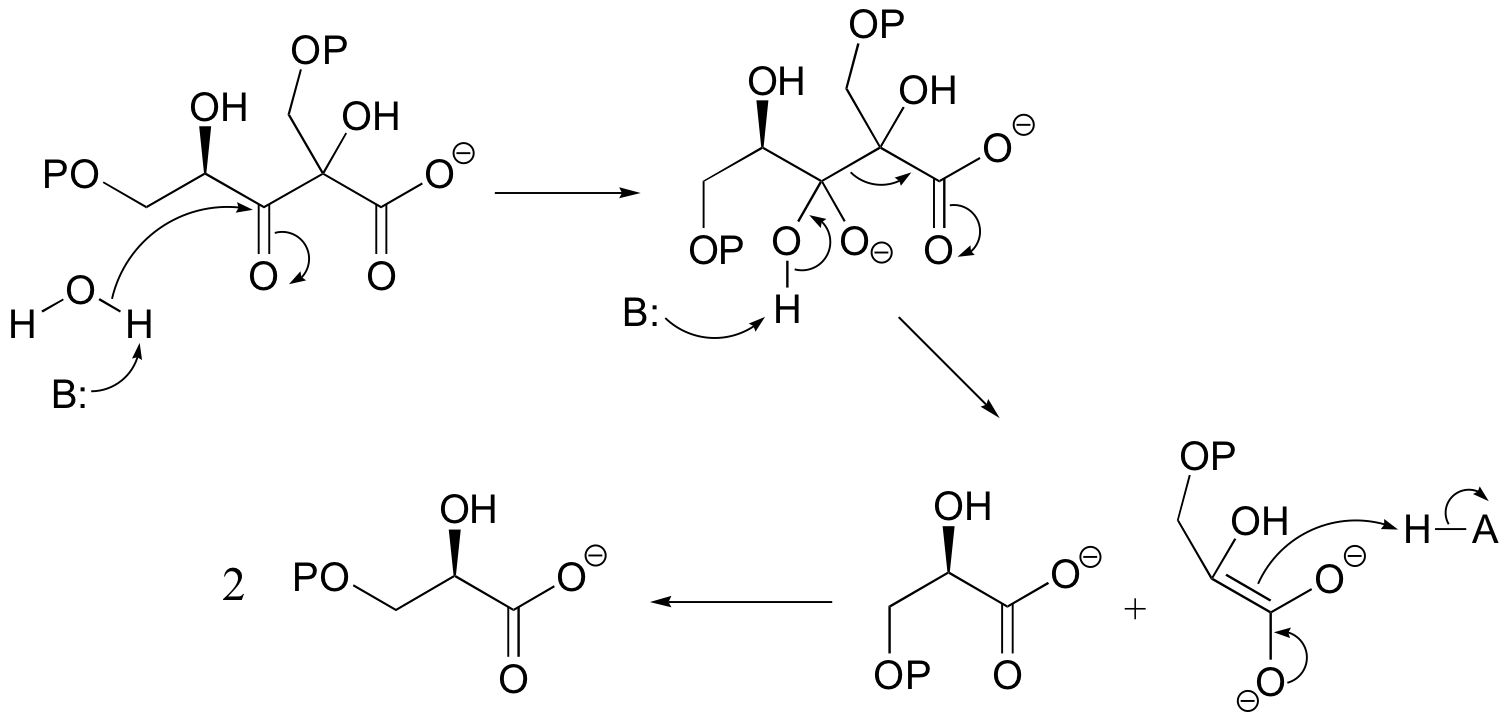
E13.9:
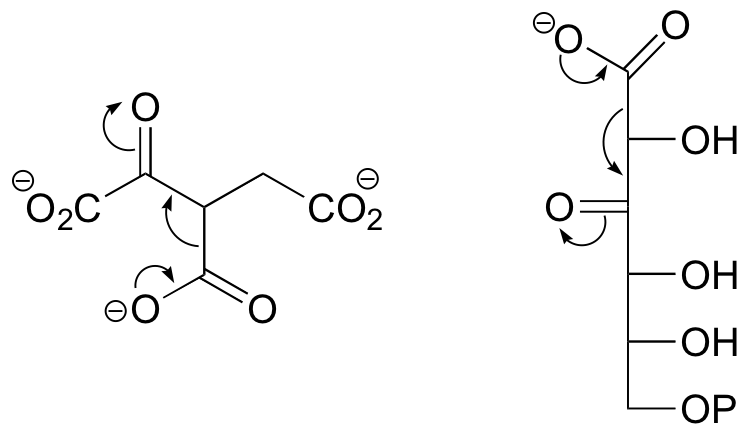
E13.10:
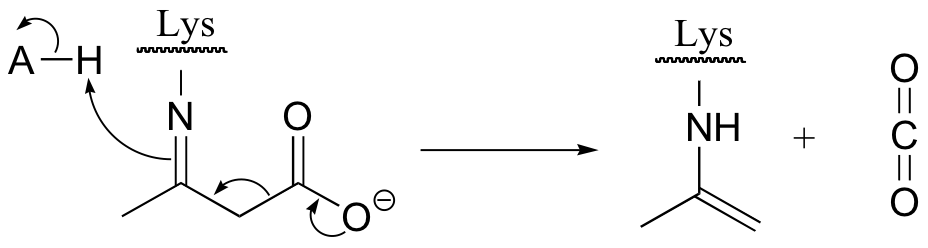
E13.11:
a)
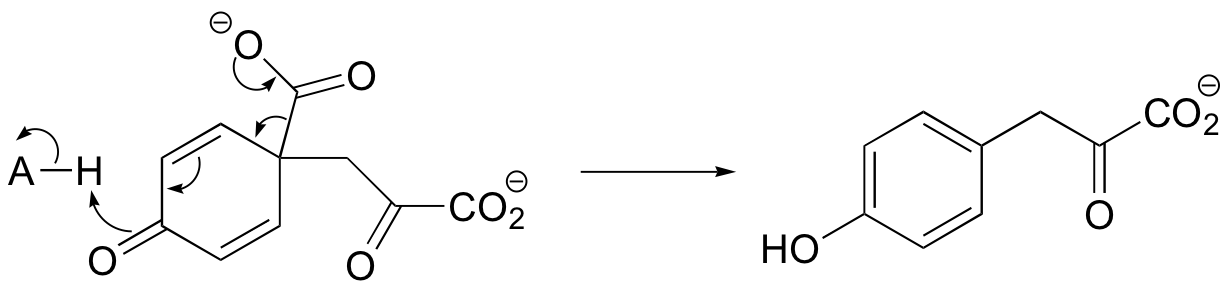
b)
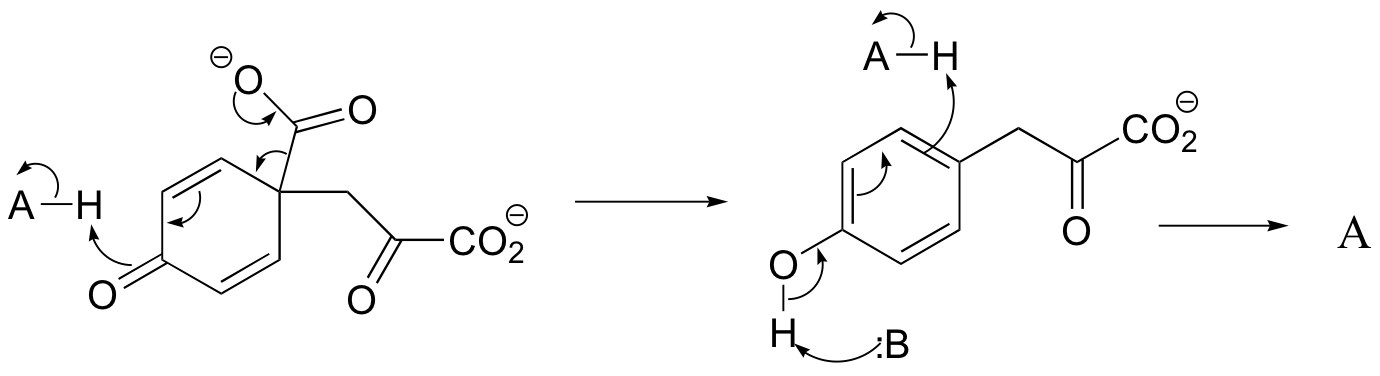
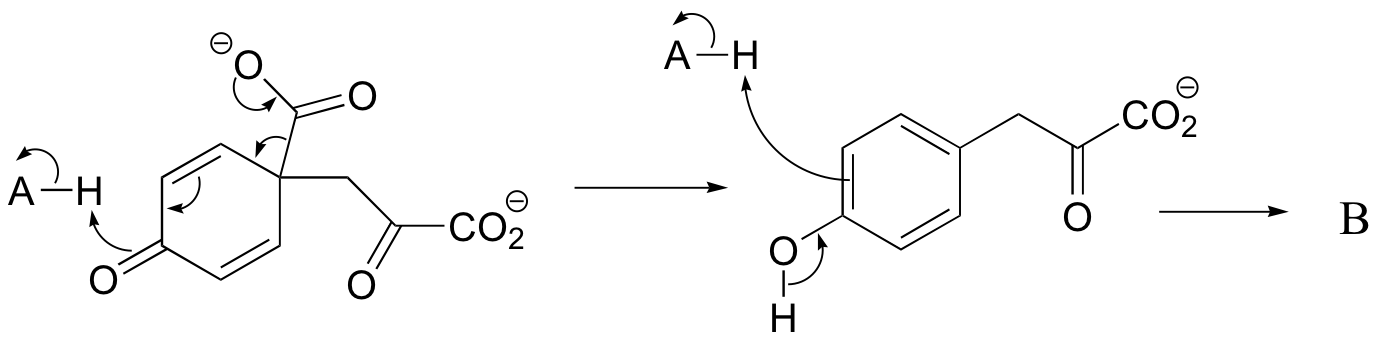
c) The relationship is tautomeric: A and B are keto forms of the actual product. Usually keto forms are more stable than enols, but in the case of phenols the enol form is aromatic and is thus much lower in energy.
E13.12:
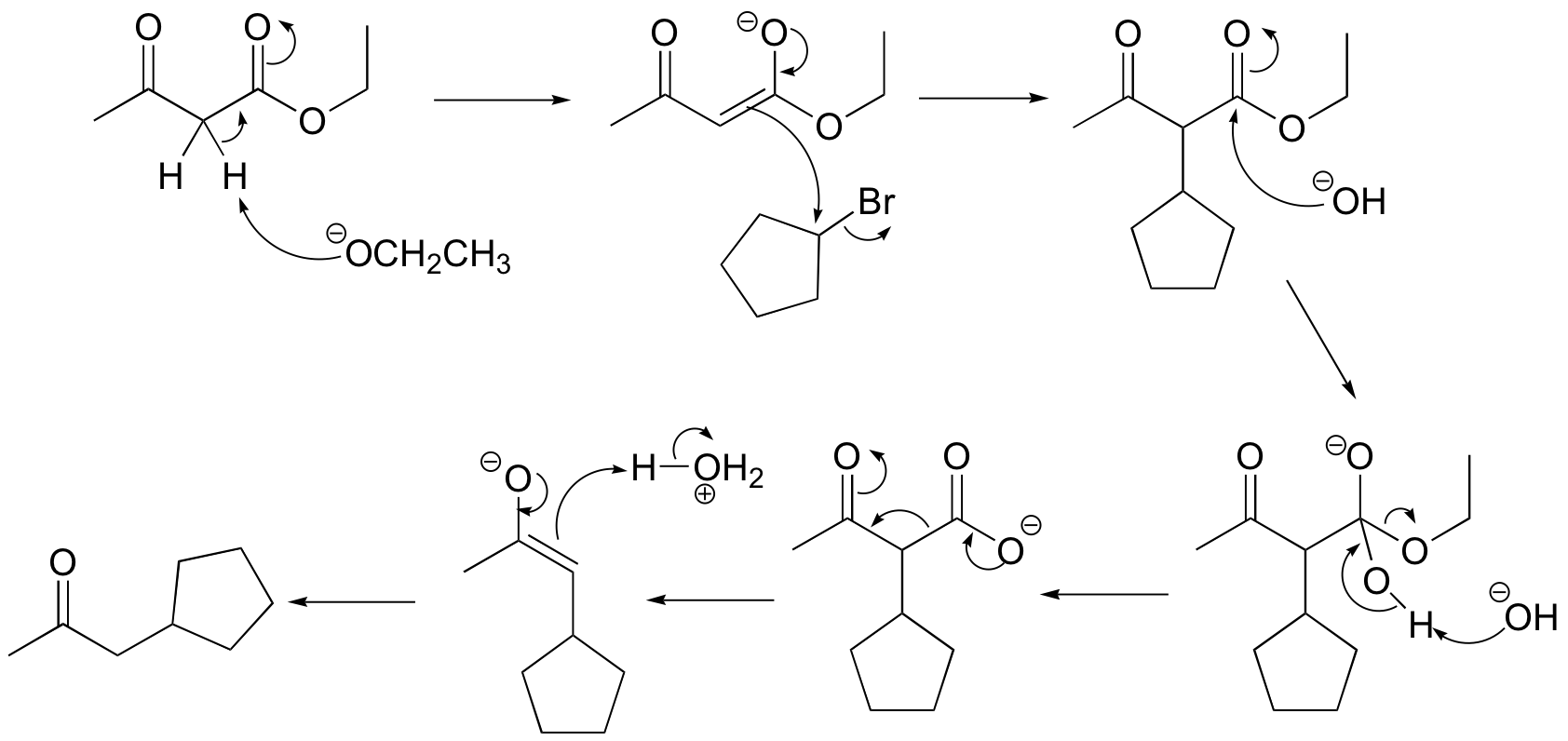
E13.13:
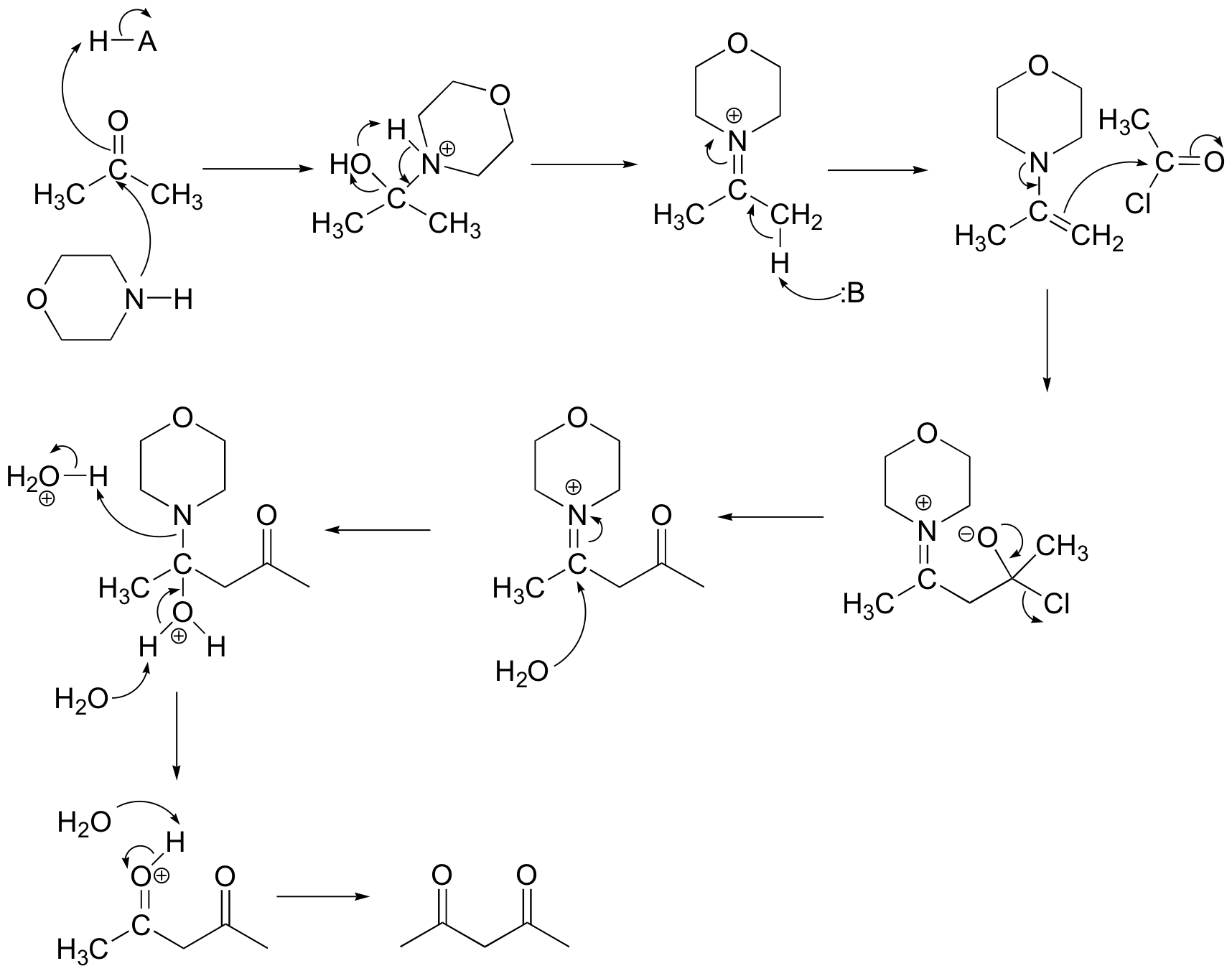
E 13.14:
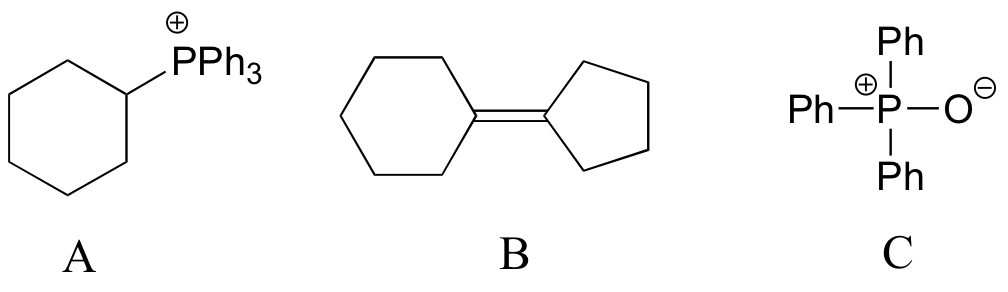
End-of-chapter problems
P13.1: Propose a mechanism for this early reaction in the biosynthesis of the isoprenoid building blocks:
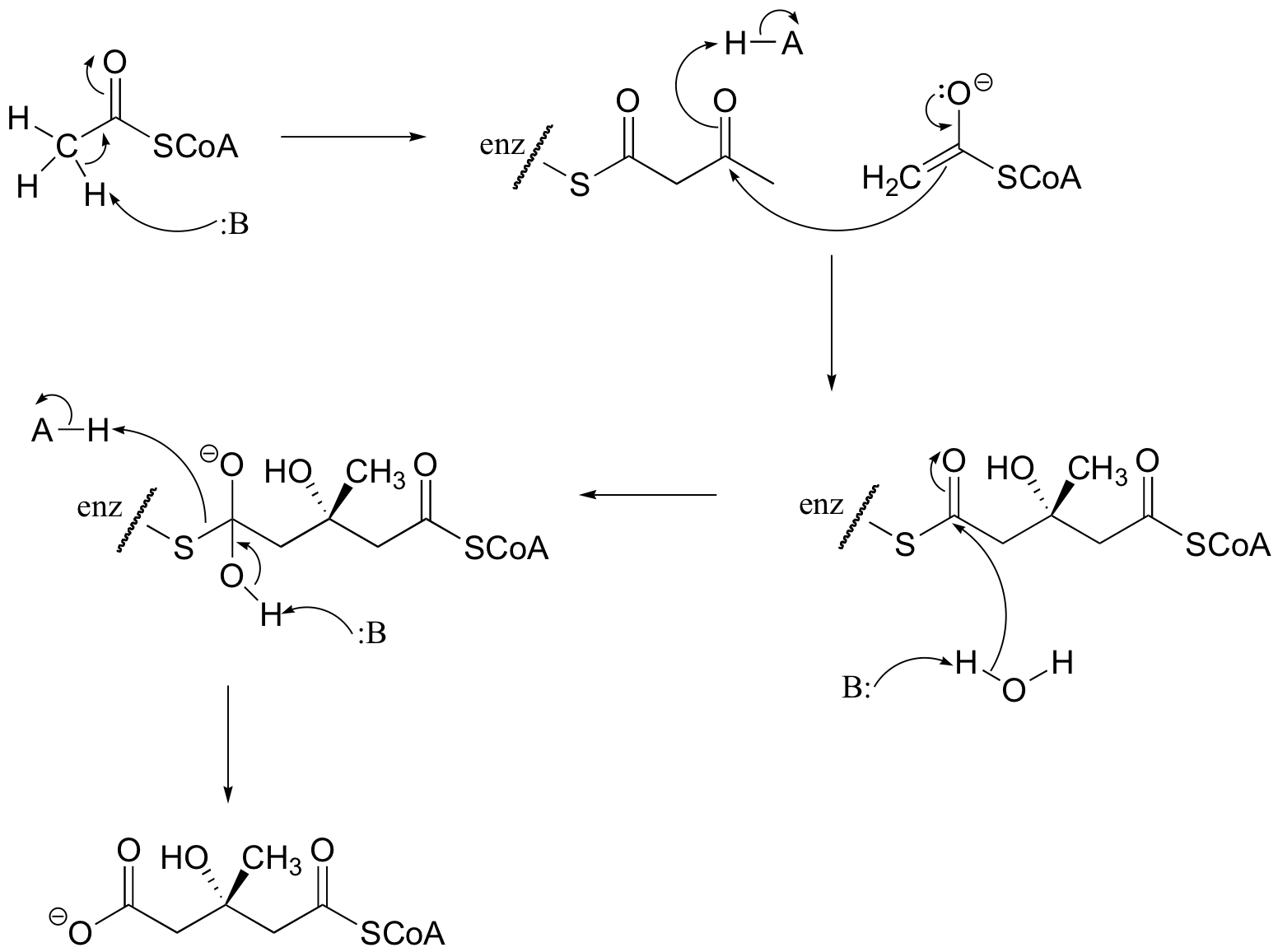
P13.2:
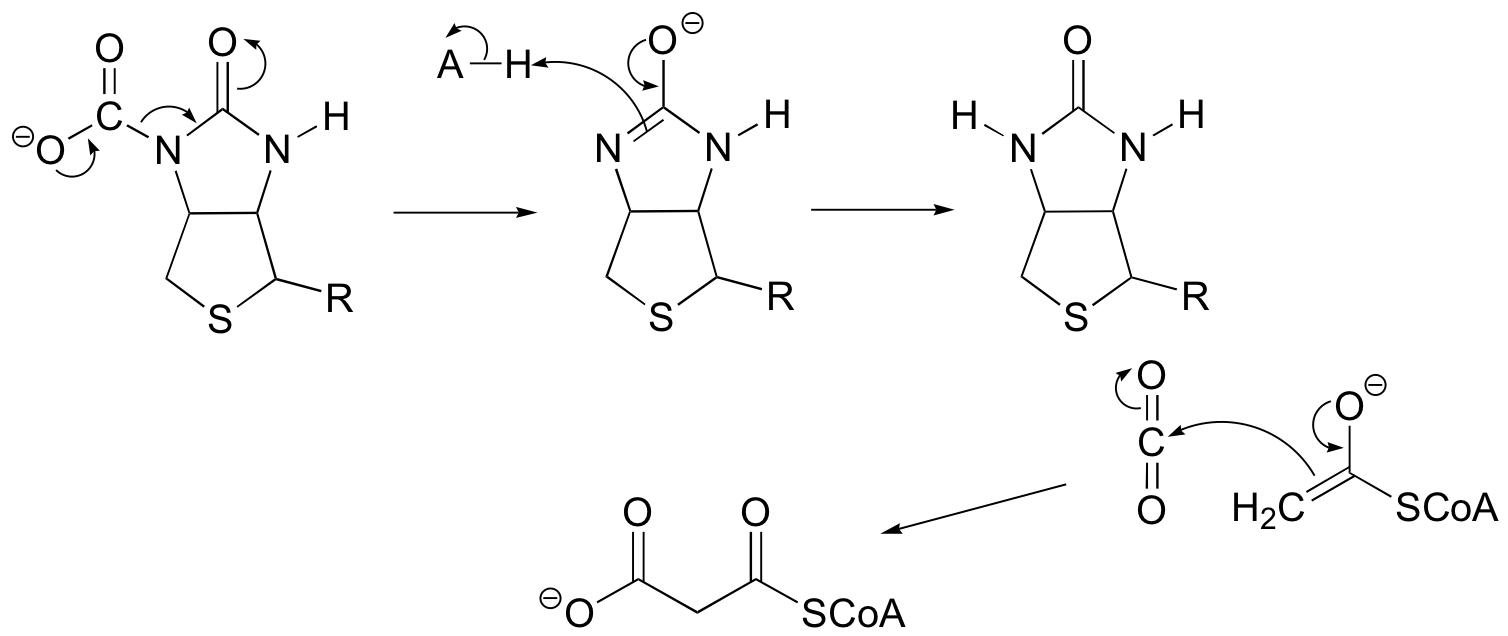
P13.3:
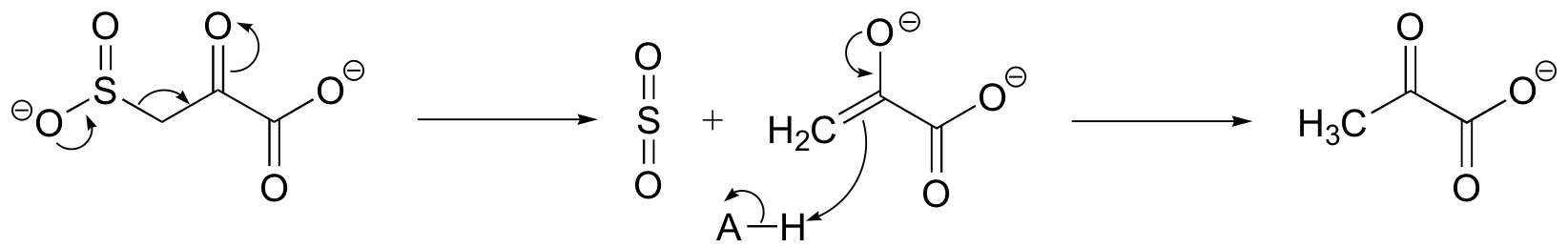
P13.4:
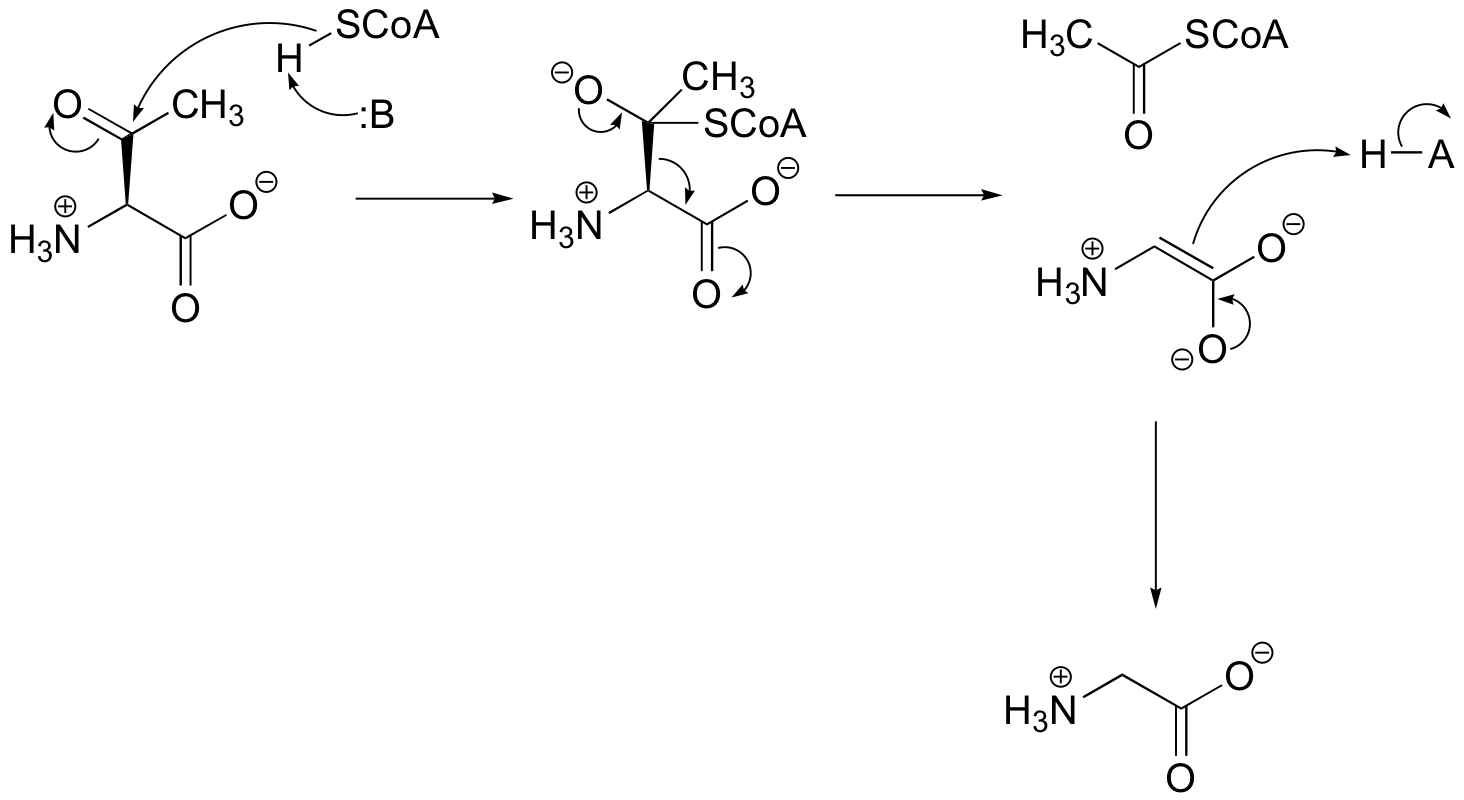
P13.5:
a)
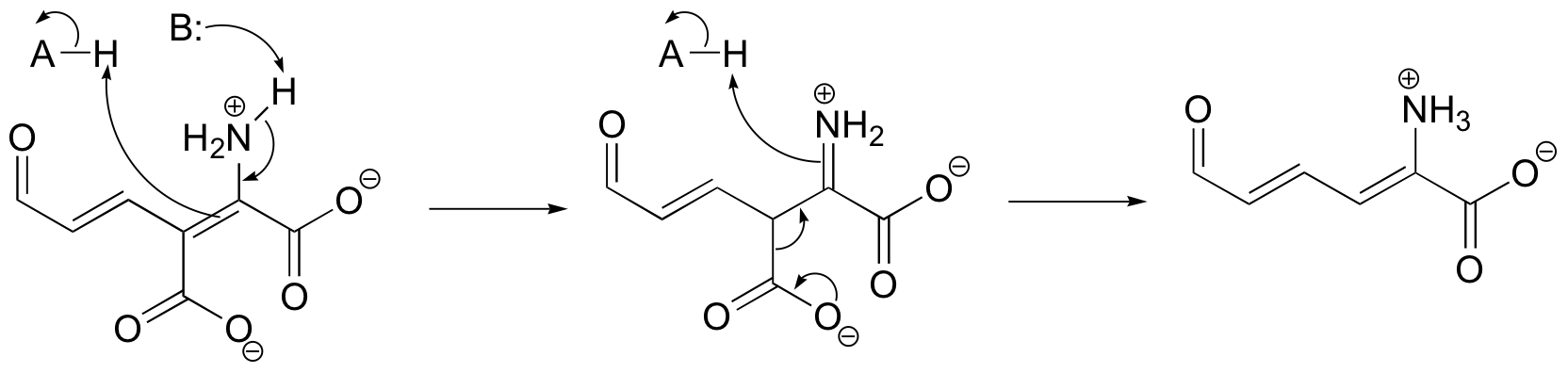
b)
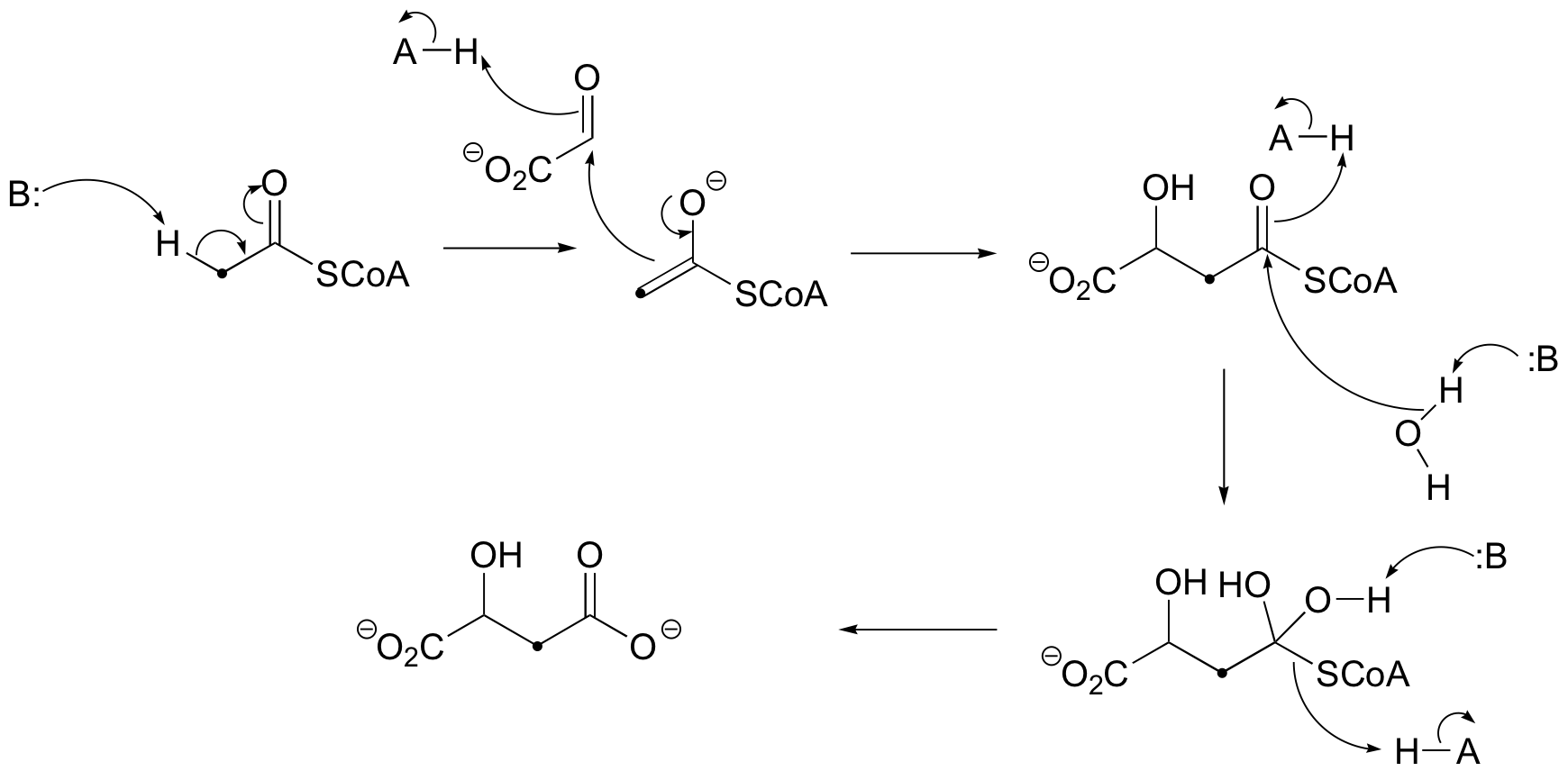
c)
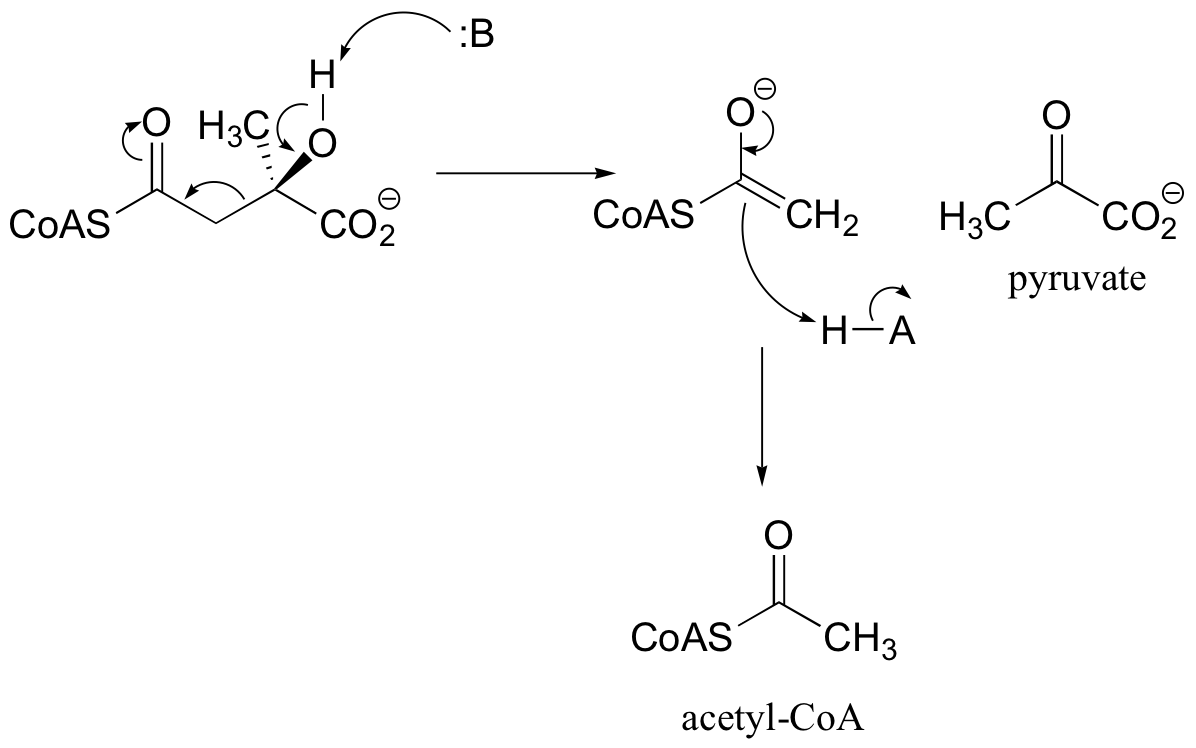
P13.6:
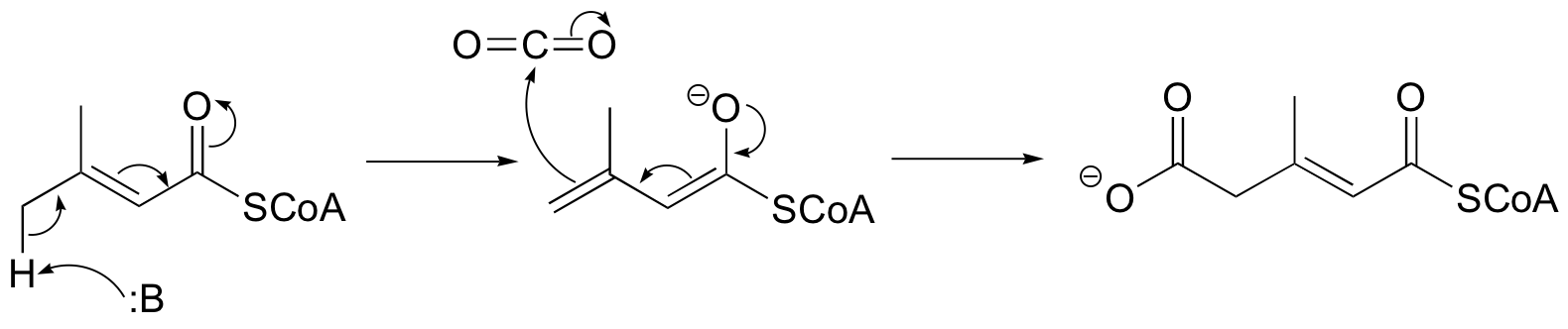
P13.7:

P13.8:
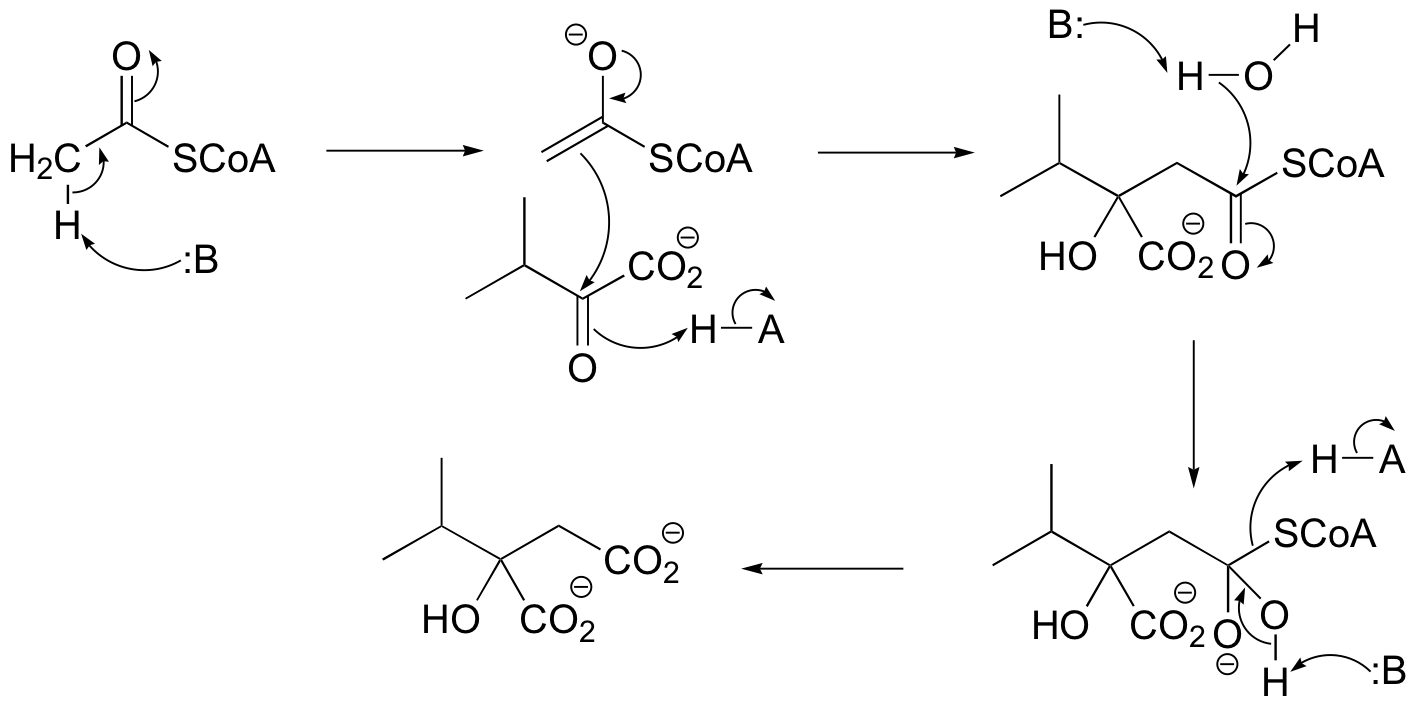
P13.9:
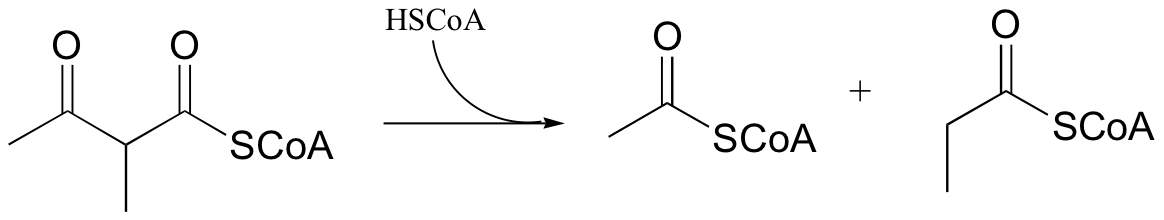
P13.10:
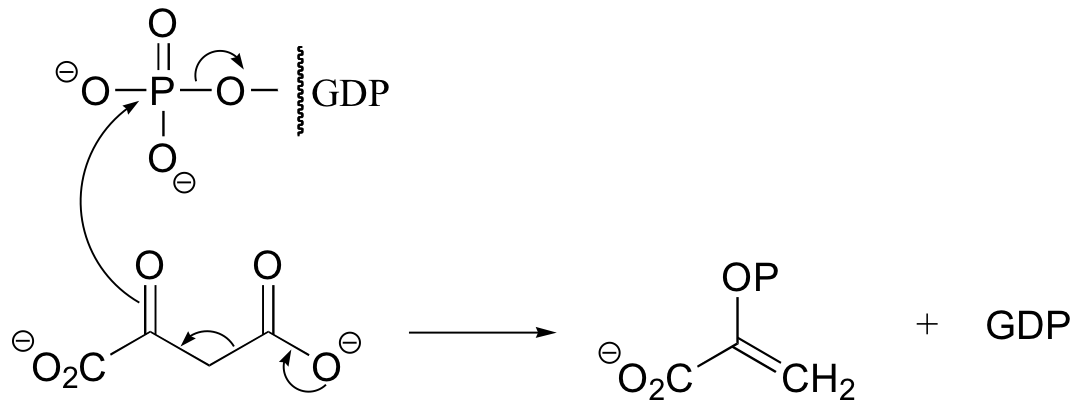
P13.11:
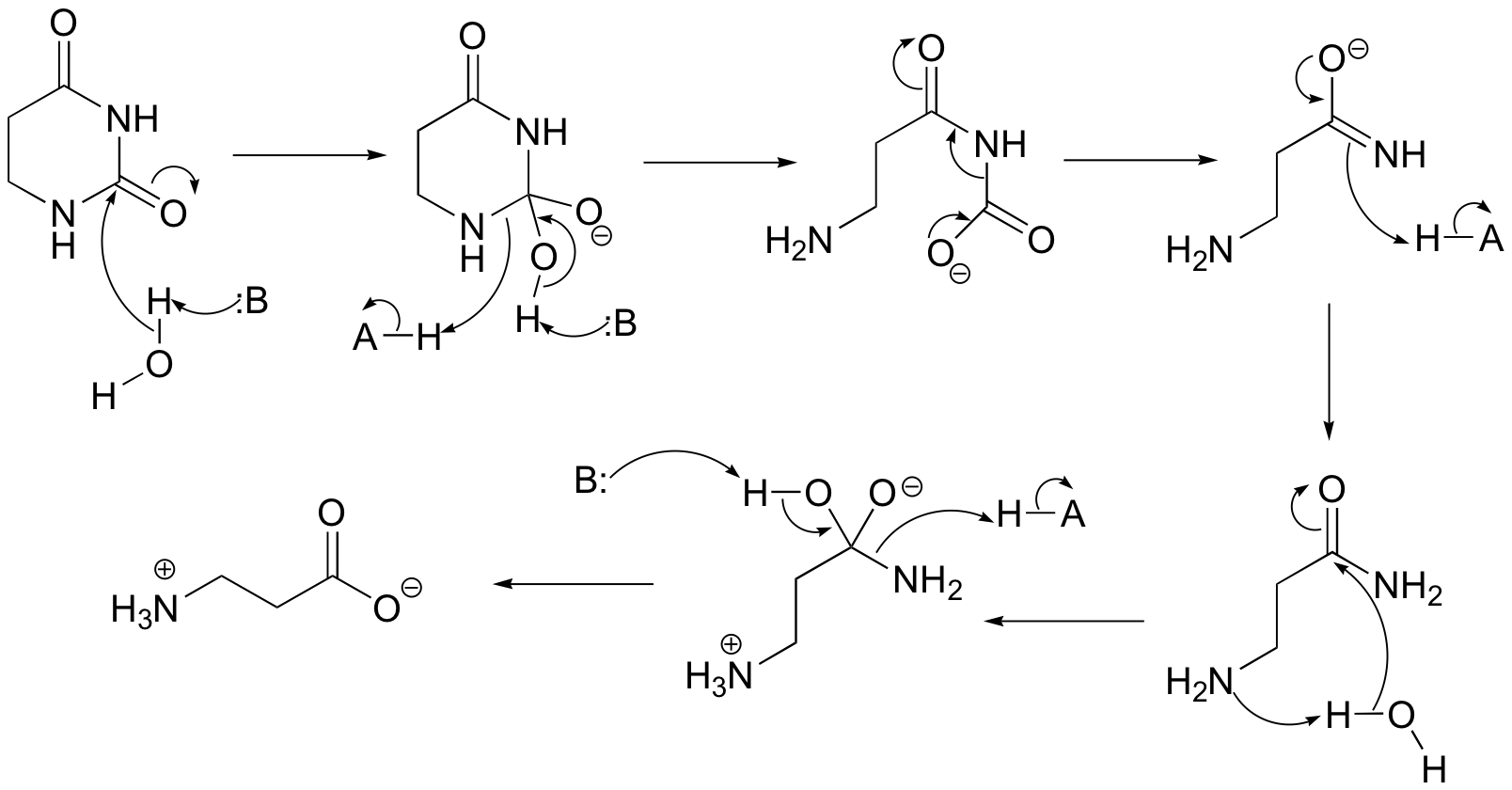
P13.12:
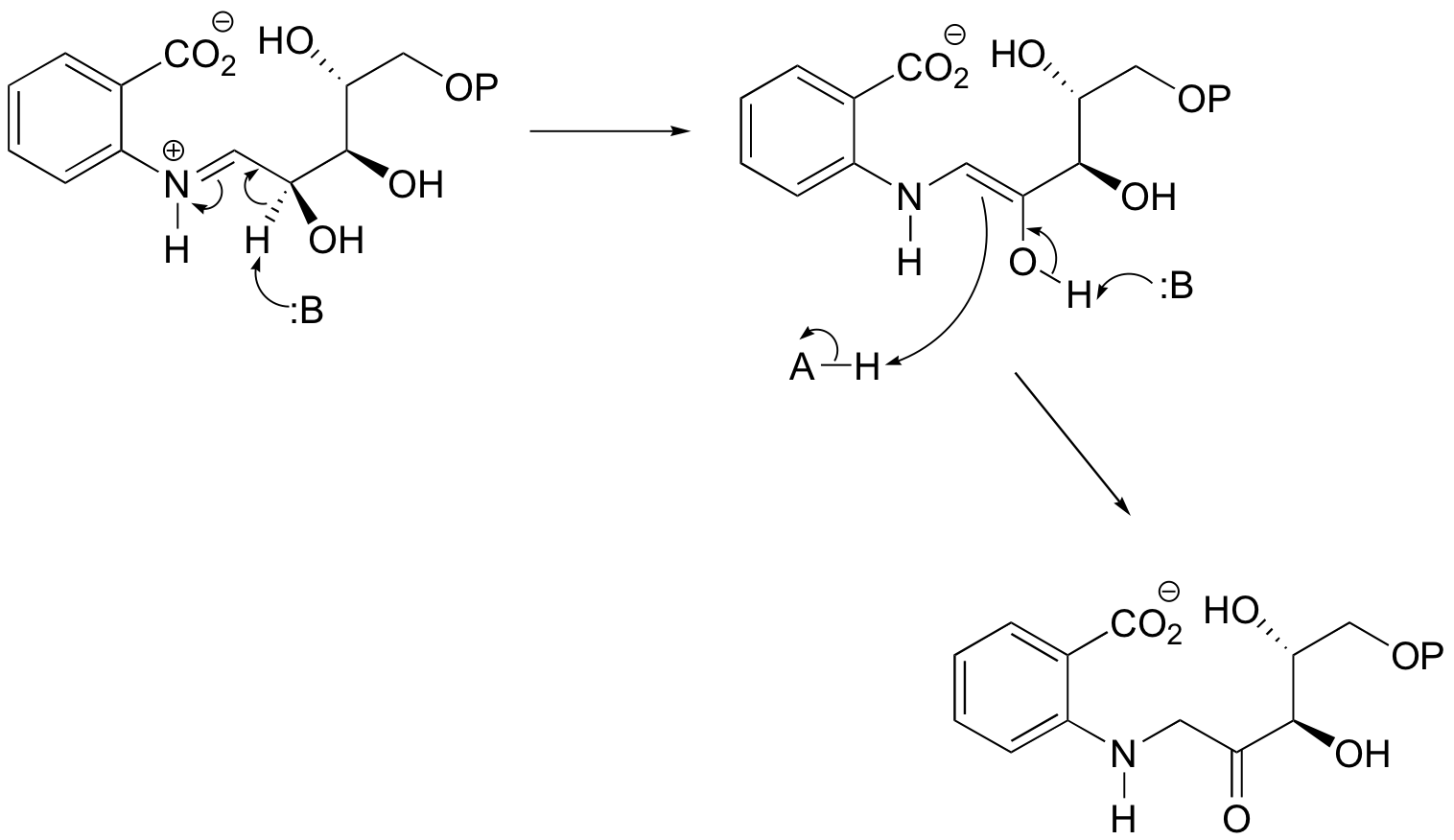
P13.13:
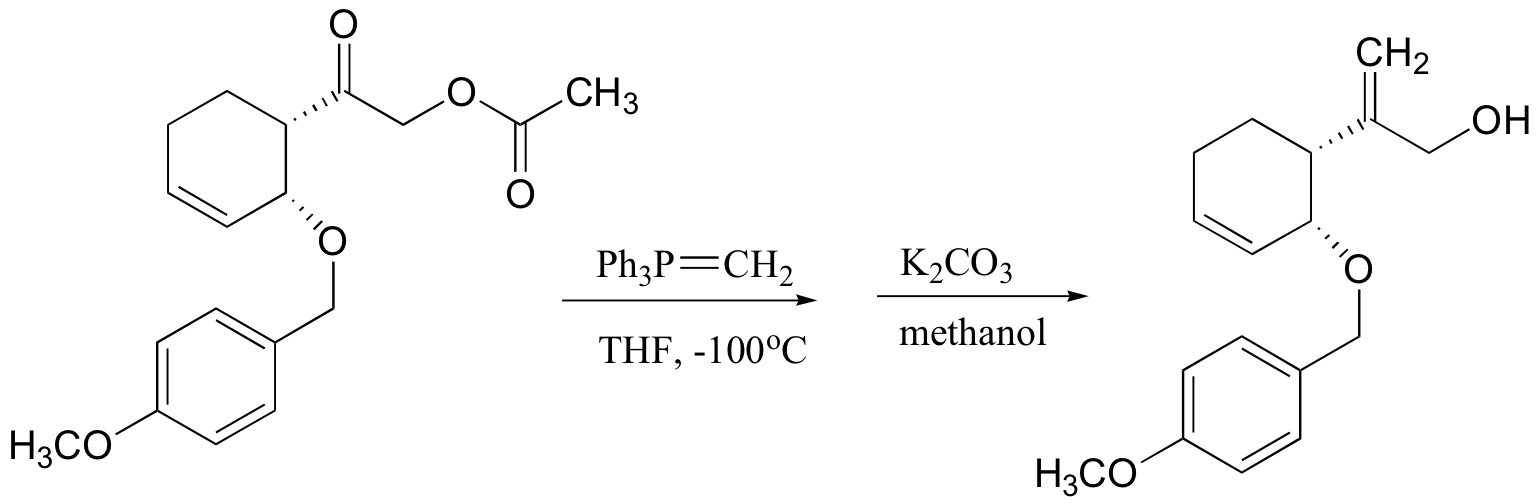
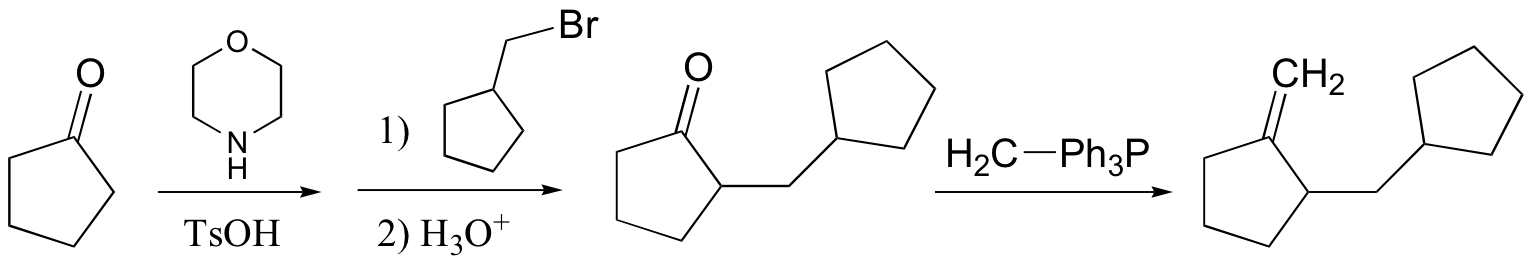
P13.14: This is a Wittig reaction, followed by a transesterification (see J. Org. Chem. 1985, 50, 3420)
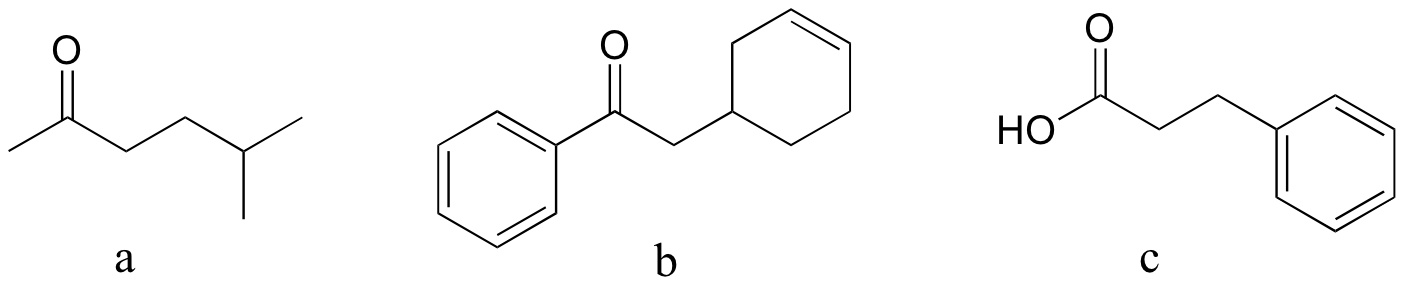
P13:15: Reactions a and b are acetoacetic ester syntheses; c is a malonic ester synthesis (section 13.6A).
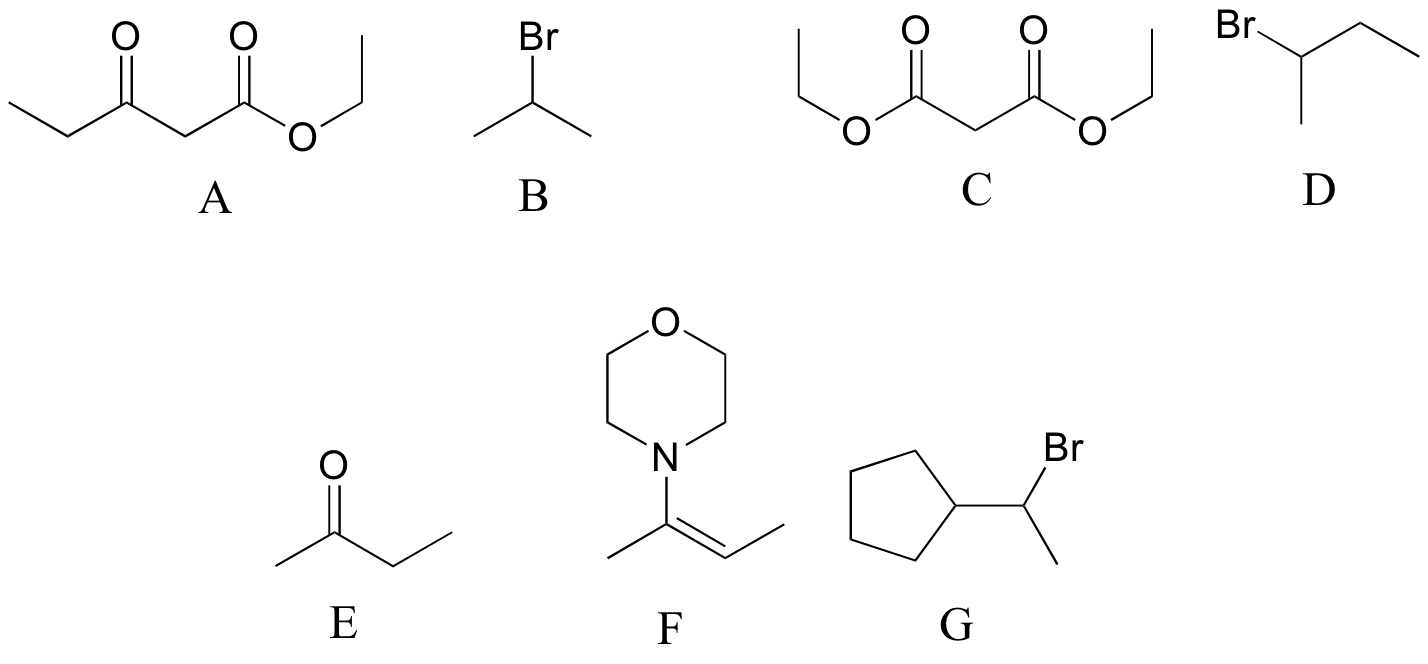
P13.16: Reaction a is an acetoacetic ester synthesis, b is a malonic ester synthesis, and c is a Stork enamine alkylation (section 13.6A)
P13.17:
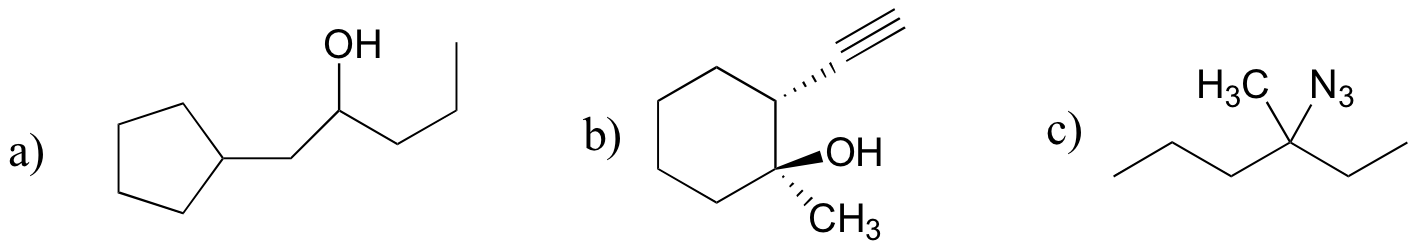
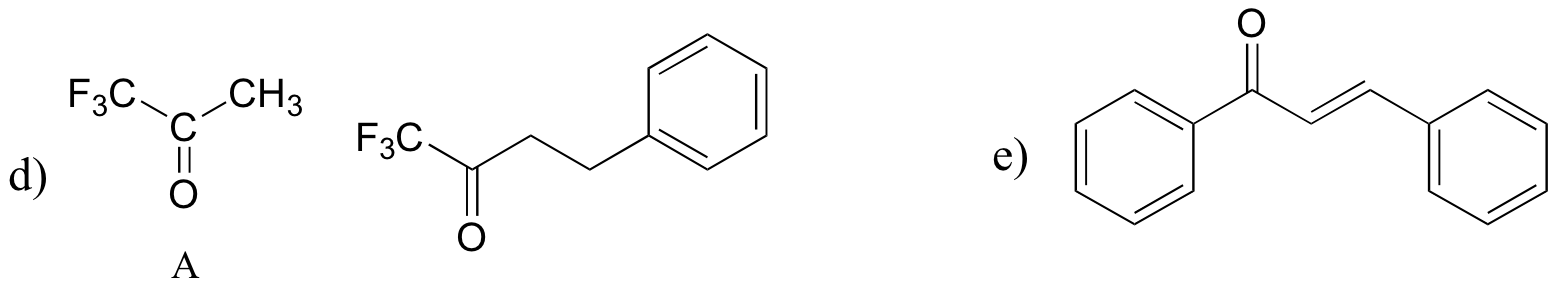
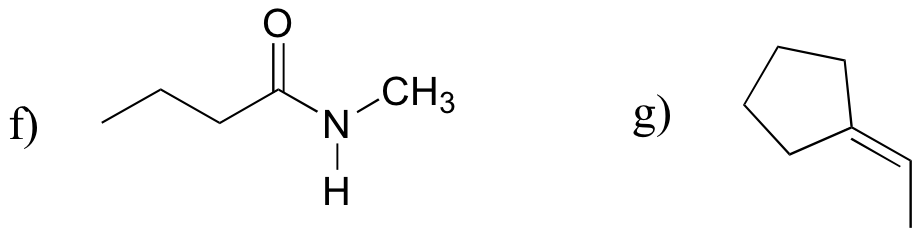
P13.18:
a)
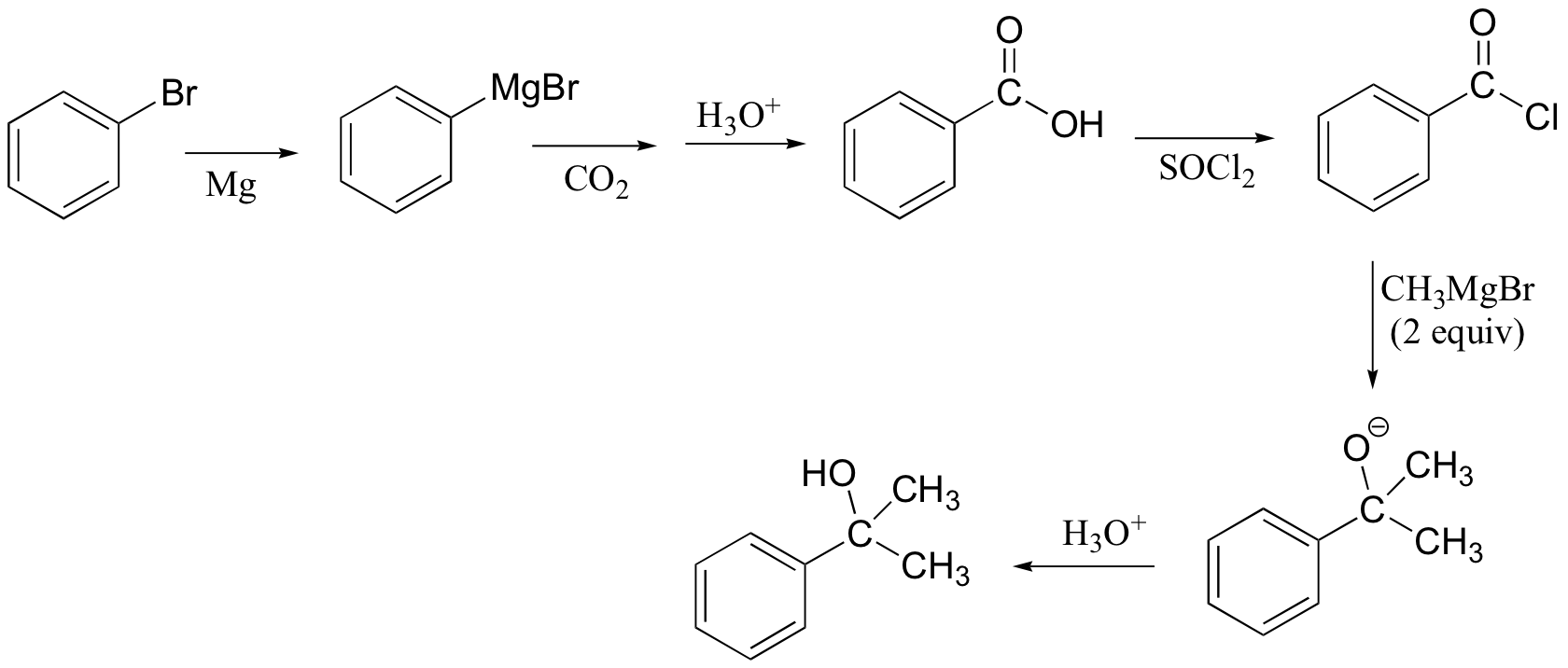
c)
Challenge problems
C13.1: