Solutions to selected chapter 3 problems
- Page ID
- 1118
P3.7:
a) Stereocenters are marked with a bold dot.
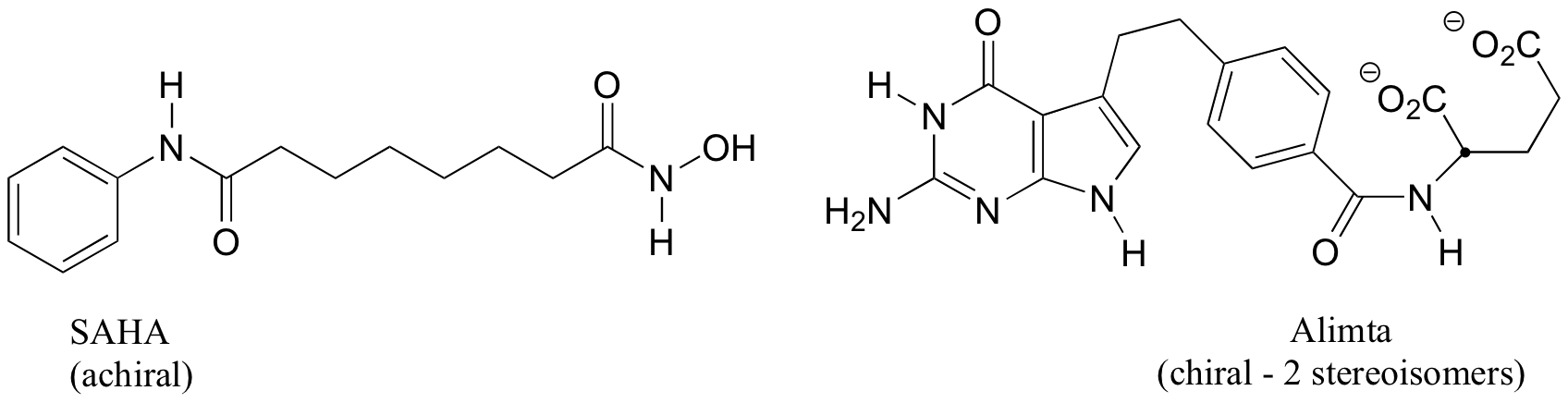
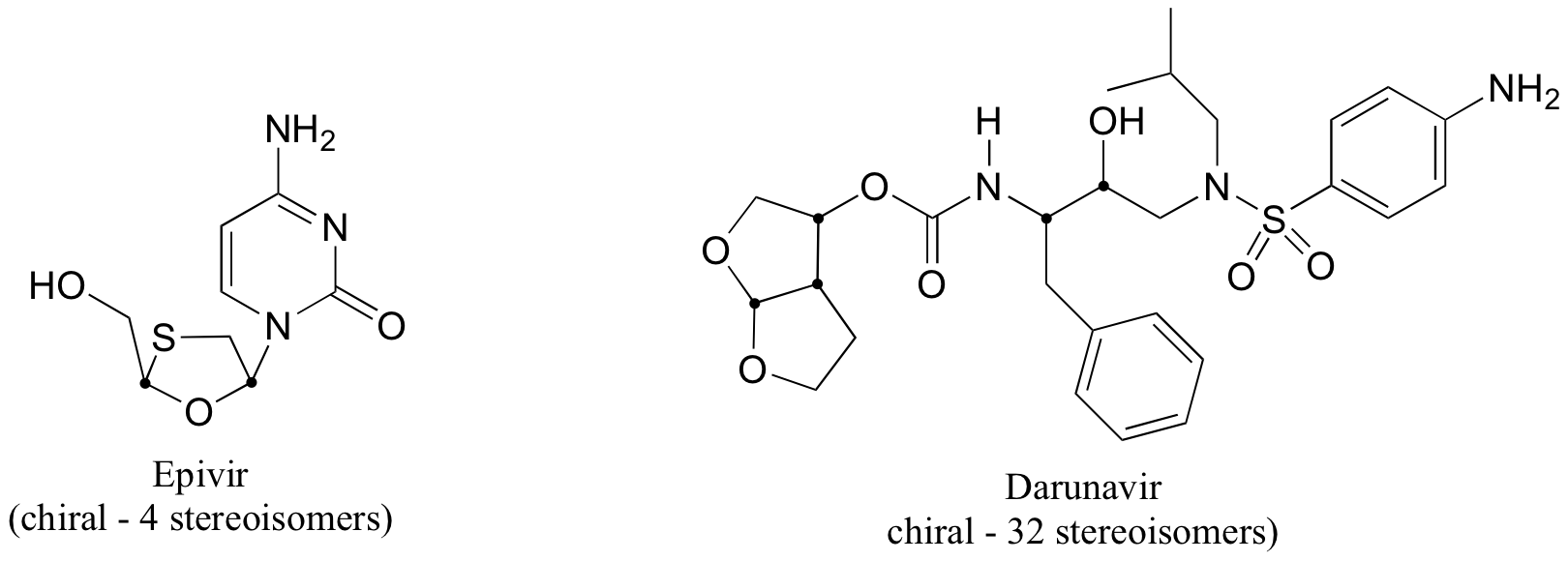
b) The two fluorinated derivatives of Epivar are enantiomers.
P3.9:
a) There is only one stereocenter, and it is in the R configuration.
b)
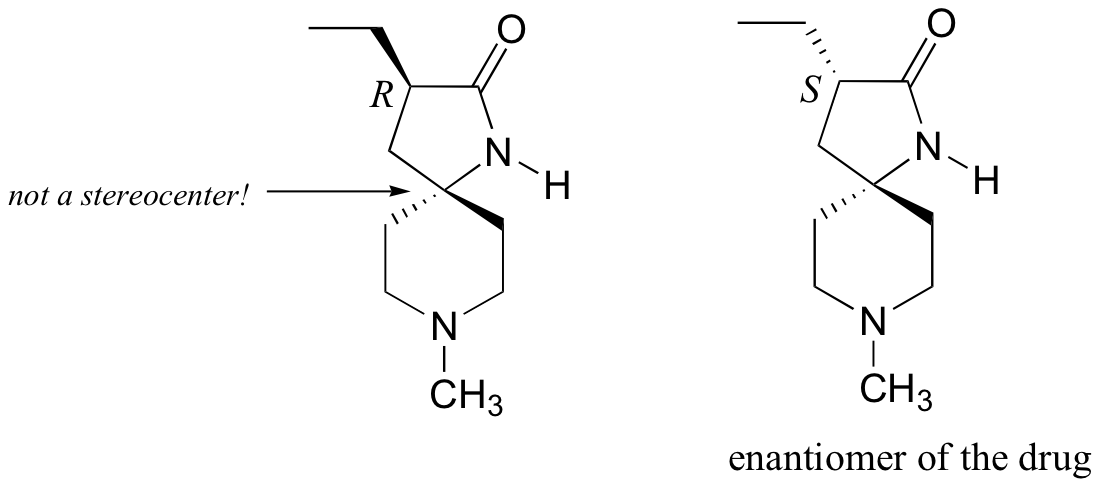
P3.10:
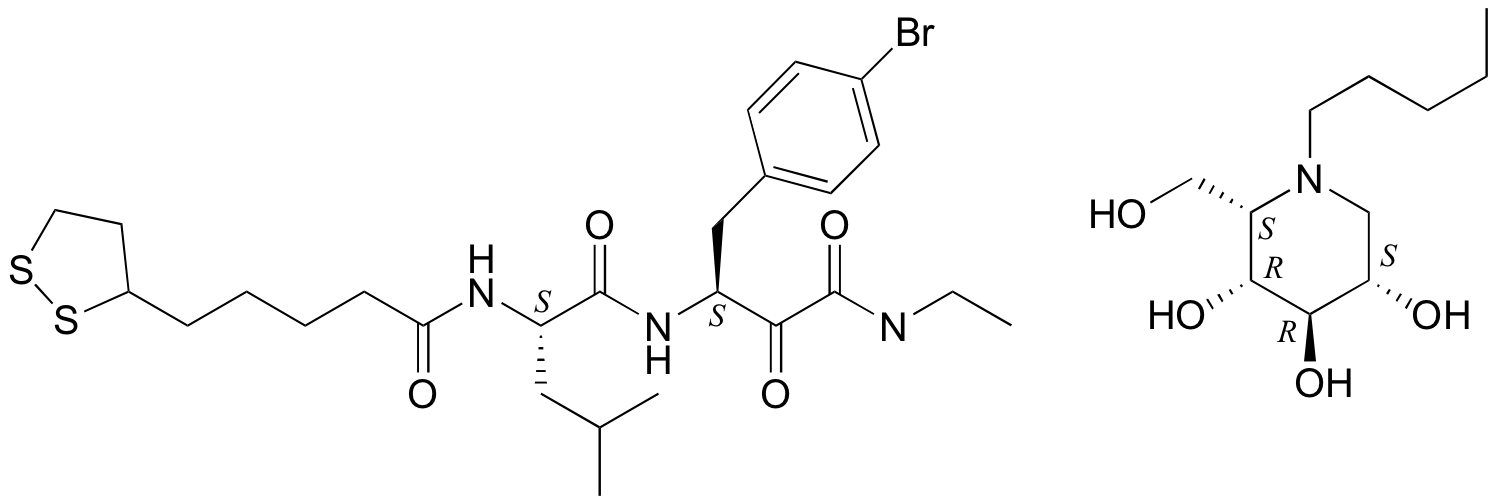
P3.11:
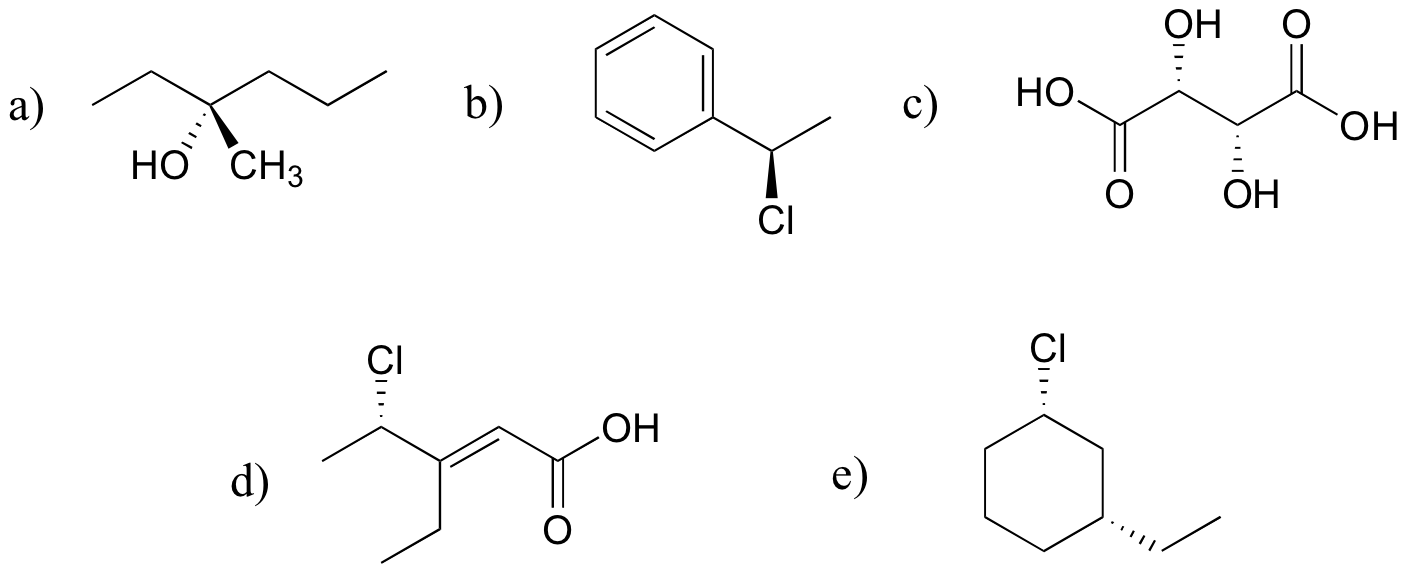
P3.12: The two are diastereomers: two of the four stereocenters are different.
P3.16:
a) The molecule contains two stereocenters and has no alkene groups that can be classified E or Z; therefore, there are four possible stereoisomers.
b) Positions of prochiral hydrogens are indicated with arrows.
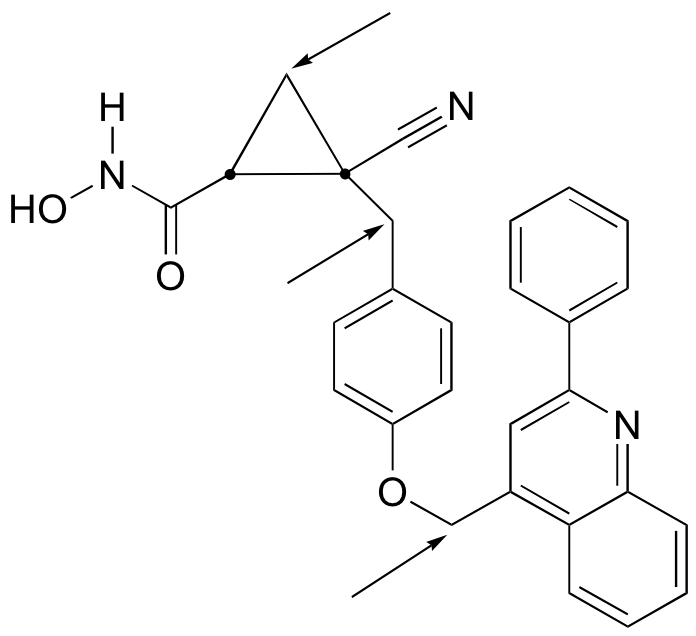
P3.17:
a) The two structures are diastereomeric.
b) The five-membered ring is sandwiched between the aromatic, seven-membered, and six-membered rings, with the ether oxygen as the free corner.
P3.18:
a) Both alkene groups are E.
b) In addition to the two alkene groups, there are 10 chiral carbons in the molecule. Therefore, there are 212 possible stereoisomers.
c) The structure shown is a diastereomer of bistramide A – the configuration of one of the alkene groups is changed from E to Z.
P3.19:
a)

b) The gauche conformation makes possible the formation of an energetically-favorable intramolecular hydrogen bond (a model will help you to see this).
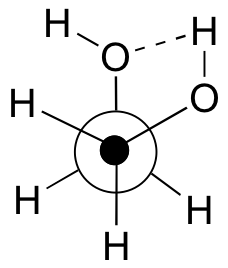
c) When the hydroxy groups are changed to methoxy groups, intramolecular hydrogen bonding is no longer possible, and the anti conformation is expected to be lowest in energy.
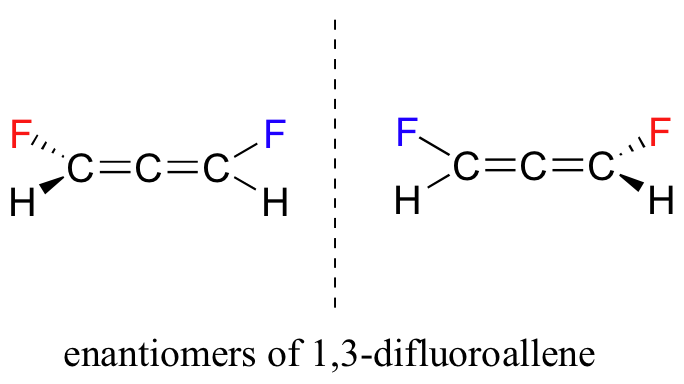
P3.21: These two structures are actually enantiomers – they are non-superimposable mirror images of each other. Notice that, even though there is no sp3-hybridized carbon, the overall geometry of the allene structure creates a multi-atom chiral center.
Contributors
- Organic Chemistry With a Biological Emphasis, Tim Soderberg (University of Minnesota, Morris)


