CX2. General Reactivity Patterns
- Page ID
- 4244
Just as a simple carbonyl is an electrophile, so is a carboxyloid. Carboxyloids react with many of the same nucleophiles that react with aldehydes and ketones. The overall result of the reaction is very different. However, the mechanism of the reaction is really quite similar.
Nucleophiles have the overall effect of adding across the carbonyl group of an aldehyde or ketone. Addition of a proton produces an alcohol. The C=O bond is parlty broken to a C-O bond. In some cases, the C=O bond is completely broken and after several steps the carbonyl oxygen is replaced by some other heteroatom. On the other hand, nucleophiles add to carboxyloids and end up replacing the heteroatom next to the carbonyl. The carbonyl itself remains intact after the reaction is complete.
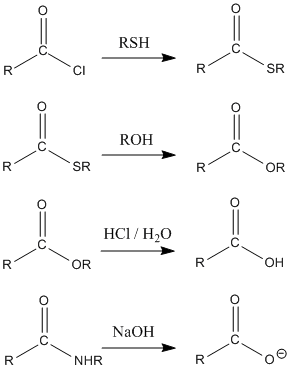
The general pattern in carboxyloid chemistry is for nucleophiles to substitute for the heteroatomic group next to the carbonyl. In these reactions, the group next to the carbonyl is sometimes referred to as a "leaving group". That term simply means that this group has been replaced by the end of the reaction.
Note that there is no reason to believe that the initial elementary reaction between a carboxyloid and a nucleophie is any different than that of a simple carbonyl with a nucleophile. An examination of the frontier orbitals in a carboxyloid suggests the pi antibonding level would be the site of population by a nucleophilic lone pair. The carbonyl pi bond would break as the nucleophile approaches.
However, an important feature of carbonyl chemistry is that two heteroatoms on one tetrahedral carbon cannot last. One always pushes the other off. If the former carbonyl oxygen pushes off the nucleophile, the system returns to the starting materials. However, if the former carbonyl oxygen displaces the heteroatomic group next to it, there is an overall change in bonding and a different product is formed. The net result is replacement of the group next to the carbonyl.
Problem CX2.1.
The overall result of reaction with a carboxyloid is to displace the heteroatom group next to the carbonyl. This group is typically liberated as an anion.
- What feature of the heteroatoms typically found attached to the carbonyl in carboxyloids allows them to be displaced from the molecule as anions?
- Why doesn't this same reaction happen with aldehydes and ketones?
Problem CX2.2.
Suggest an order of reactivity for the carboxyloids: rank them from most reactive to least reactive. Provide a reason for your trend.