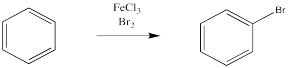AR2. Mechanism of EAS
- Page ID
- 4276
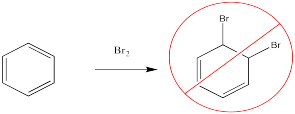
Bromine will not add across the double bond of benzene.
Instead, a bromine atom can replace one of the hydrogen atoms on the benzene. This reaction is especially easy in the presence of a catalyst.
How does that outcome happen? Why does that outcome happen?
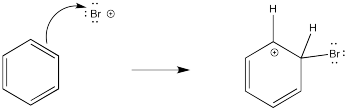
There has been a good deal of study of these reactions and there is strong evidence of the steps through which they occur. As expected, the reaction involves donation of π electrons from the benzene. For the moment, we'll assume the electrophile is a bromine cation; we will deal with its exact structure later.
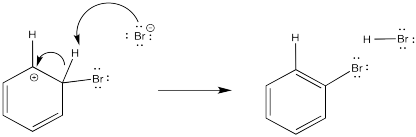
The problem is, that initial step results in the loss of aromaticity. The aromatic system confers a little extra stability on the π system, so the molecule is motivated to restore the aromaticity. The easiest way to do that, and get rid of a positive charge at the same time, would be to deprotonate the cation. Some base will pick up the proton; it is likely a bromide ion in this case. We will see later where that bromide comes from.
- Electrophilic aromatic substitution proceeds through a cationic intermediate.
- The intermediate forms via donation of π electrons from the arene to an electrophile.
- Aromaticity is restored through loss of a proton from this cation.
How do we know that the mechanism unfolds this way? There are three basic steps that are clearly accomplished during the course of the reaction: the C-H bond is broken, the C-Br bond is formed, and the Br-Br bond is broken.
When is the C-H bond broken? That question can be answered by looking for what is called an "isotope effect." The most common isotope of hydrogen is 1H, or protium, but 2H is also available; it is called "deuterium." Deuterium is often represented by the symbol D and protium by the symbol H. Deuterium is twice as heavy as the common protium. That mass difference leads to a lower vibrational frequency of a C-D bond than a C-H bond. The C-H bond vibrates more rapidly and energetically than a C-D bond; as a consequence, the C-H bond is more easily broken than the C-D bond.
If we take a sample of ordinary benzene, C6H6, and a sample of deuterated benzene, C6D6, we can measure how quickly they each undergo a bromination reaction. Very often, a reaction that involves C-H bond cleavage will slow down if a C-D bond is involved. This outcome is observed in E2 eliminations, for instance. This slowing of the reaction with the heavier isotope is called the deuterium isotope effect.
However, no deuterium isotope effect is observed during bromination, or other aromatic electrophilic substitution reactions. That absence of an isotope effect usually means the C-H bond cleavage is a sort of an afterthought. The hard part of the reaction is already done. Both the C-H and C-D bonds are broken so quickly and easily, by comparison, that we don't really notice the difference between them.
There is even more evidence. In a few exceptional cases, the cationic intermediate in this reaction is stable enough to be isolated and crystallized. X-ray diffraction shows that there is a tetrahedral carbon in the ring, indicating that the C-H bond has not broken yet.
The C-H bond is broken at the end of the reaction. When is the Br-Br bond broken?
That question is a little harder to answer. We can't use the same isotope strategy that we used with the C-H bond. Although deuterium is twice as heavy as protium, producing a substantial isotope effect, 81Br is only 2.5% more massive than 79Br. Any difference in rates involving these isotopes is undetectable. The exact nature of the bromine species in the reaction is complicated, and may even be different under different conditions.
Problem AR2.1.
In the case of uncatalyzed bromination reactions, the addition of salts such as NaBr has no effect on the reaction rate, indicating that the arene reacts directly with Br2 rather than Br+. Explain this line of reasoning.
Problem AR2.2.
Given each of the following electrophiles, provide a mechanism for electrophilic aromatic substitution.
a) NO2+ b) CH3CH2+ c) SO3H+ d) CH3CO+