Dieckmann Condensation
- Page ID
- 66507
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation.
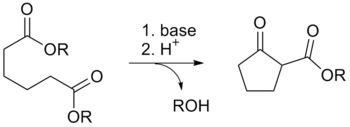
Reaction Mechanism
Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Bronsted-Lowry acid (H3O+ for example) re-forms the β-keto ester.[6]
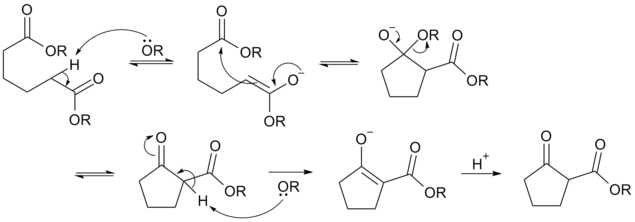
Owing to the steric stability of five- and six-membered ring structures, these will preferentially be formed. So 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters.[7]
Animation of the reaction mechanism
See also
- Claisen condensation
- Gabriel-Colman rearrangement
References
- Dieckmann, W. Ber. 1894, 27, 102 & 965
- Dieckmann, W. Ber. 1900, 33, 595 & 2670
- Dieckmann, W. Ann. 1901, 317, 51 & 93
- Schaefer, J. P.; Bloomfield, J. J. (1967). "The Dieckmann Condensation (Including the Thorpe-Ziegler Condensation)". Organic Reactions. 15: 1–203. doi:10.1002/0471264180.or015.01.
- Davis, B. R.; Garrett, P. J. Comp. Org. Syn. 1991, 2, 806-829. (Review)
- Janice Gorzynski Smith (2007). Organic Chemistry (2nd ed.). pp. 932–933. ISBN 978-0073327495.
- "Dieckmann Condensation". Organic Chemistry Portal.
Contributors
Wikipedia (CC-BY-SA-3.0)