Symmetry and Point Groups
- Page ID
- 1262
The symmetry of a molecule is determined by the existence of symmetry operations performed with respect to symmetry elements. A symmetry element is a line, a plane or a point in or through an object, about which a rotation or reflection leaves the object in an orientation indistinguishable from the original. A plane of symmetry is designated by the symbol σ (or sometimes s), and the reflection operation is the coincidence of atoms on one side of the plane with corresponding atoms on the other side, as though reflected in a mirror. A center or point of symmetry is labeled i, and the inversion operation demonstrates coincidence of each atom with an identical one on a line passing through and an equal distance from the inversion point (see chair cyclohexane). Finally, a rotational axis is designated Cn, where the degrees of rotation that restore the object is 360/n (C2= 180º rotation, C3= 120º rotation, C4= 90º rotation, C5= 72º rotation). C1 is called the identity operation E because it returns the original orientation.
An object having no symmetry elements other than E is called asymmetric. Such an object is necessarily chiral. Since a plane or point of symmetry involves a reflection operation, the presence of such an element makes an object achiral. One or more rotational axes of symmetry may exist in both chiral, dissymmetric, and achiral objects.
Three dimensional models illustrating these symmetry elements will be displayed on the right by clicking one of the following names. The forth and seventh of these are dissymmetric. The others are achiral.
Examples
- cis-1,2-Dichloroethene
- trans-1,2-Dichloroethene
- cis-1,2-Dimethylcyclopropane
- trans-1,2-Dimethylcyclopropane
- Cyclohexane (chair conformer)
- Cyclohexane (boat conformer)
- Cyclohexane (twist boat conformer)
- Allene
- 1,3,5,7-Tetrafluoro-1,3,5,7-Cyclooctatetraene
One more symmetry operation must be defined. Both trans-dimethylcyclopropane and 1,3,5,7-tetrafluoro-1,3,5,7-cyclooctatetraene have a C2 axis, and both lack a plane or center of symmetry. The former is chiral, but the latter is achiral because it has a S4 improper rotational axis (sometimes called an alternating axis). An improper axis, Sn, consists of a n-fold rotation followed by reflection through a mirror plane perpendicular to the rotation axis (n is always 3 or larger because S1 = σ and S2 = i). This is equivalent to saying that a n-fold rotation converts an object into its mirror image.
The S4 element in 1,3,5,7-tetrafluoro-1,3,5,7-cyclooctatetraene will be illustrated above by Clicking Here.
Symmetry Point Groups
An object may be classified with respect to its symmetry elements or lack thereof. This is done by assigning a symmetry point group, reflecting the combination of symmetry elements present in the structure. For example, bromochlorofluoromethane has no symmetry element other than C1 and is assigned to that point group. All C1 group objects are chiral. Other low symmetry point groups are Cs (only a single plane of symmetry) and Ci (only a point of symmetry). Objects in either of these point groups are achiral.
Some objects are highly symmetric and incorporate many symmetry elements. Methane is an example of a high symmetry molecule, having 4 C3 axes, 3 C2 axes and 6 σ (planes); it belongs to the tetrahedral point group Td. When combinations of rotational axes and planes are present, their relationship is designated by a v (vertical), h (horizontal) or d (diagonal). Thus, a plane containing the principle rotation axis is σv, a plane perpendicular to the principle rotation axis is σh, and a plane parallel to the principle rotation axis but bisecting the angle between two C2 axes is σd. By this notation, the six planes of the methane tetrahedron are all σd. Some of the symmetry elements of methane will be shown below by Clicking Here.
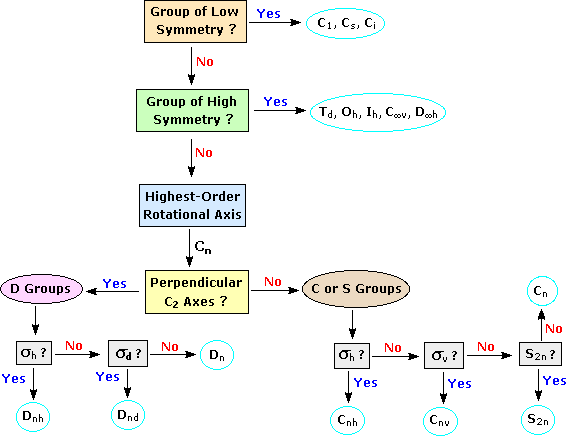
Objects of intermediate symmetry may be assigned to appropriate point groups by following the decision tree shown below. For example, trans-1,2-dichloroethene, which has a C2 axis perpendicular to its single plane of symmetry, belongs to the C2h point group. By clicking on any of the nine categories circled in light blue, further examples will be provided.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


