Practice Problems
- Page ID
- 1257
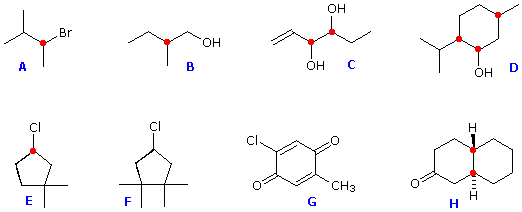
Structural formulas for eight organic compounds are displayed in the frame below. Some of these structures are chiral and some are achiral. First, try to identify all chiral stereogenic centers. Formulas having no chiral centers are necessarily achiral. Formulas having one chiral center are always chiral; and if two or more chiral centers are present in a given structure it is likely to be chiral, but in special cases, to be discussed later, may be achiral.
AnswerS
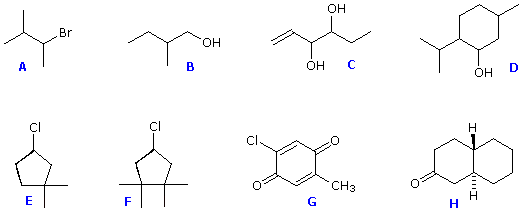
Structures F and G are achiral. The former has a plane of symmetry passing through the chlorine atom and bisecting the opposite carbon-carbon bond. The similar structure of compound E does not have such a symmetry plane, and the carbon bonded to the chlorine is a chiral center (the two ring segments connecting this carbon are not identical). Structure G is essentially flat. All the carbons except that of the methyl group are sp2 hybridized, and therefore trigonal-planar in configuration. Compounds C, D & H have more than one chiral center, and are also chiral. Remember, all chiral structures may exist as a pair of enantiomers. Other configurational stereoisomers are possible if more than one stereogenic center is present in a structure.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry