Reactions of Fused Benzene Rings
- Page ID
- 1206
Compounds in which two or more benzene rings are fused together were described in an earlier section, and they present interesting insights into aromaticity and reactivity. The smallest such hydrocarbon is naphthalene. Naphthalene is stabilized by resonance. Three canonical resonance contributors may be drawn, and are displayed in the following diagram.
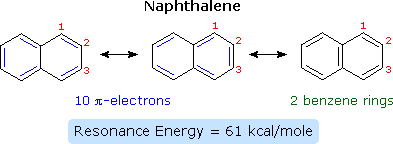
The two structures on the left have one discrete benzene ring each, but may also be viewed as 10-pi-electron annulenes having a bridging single bond. The structure on the right has two benzene rings which share a common double bond. From heats of hydrogenation or combustion, the resonance energy of naphthalene is calculated to be 61 kcal/mole, 11 kcal/mole less than that of two benzene rings (2 * 36). As expected from an average of the three resonance contributors, the carbon-carbon bonds in naphthalene show variation in length, suggesting some localization of the double bonds. The C1–C2 bond is 1.36 Å long, whereas the C2–C3 bond length is 1.42 Å. This contrasts with the structure of benzene, in which all the C–C bonds have a common length, 1.39 Å.
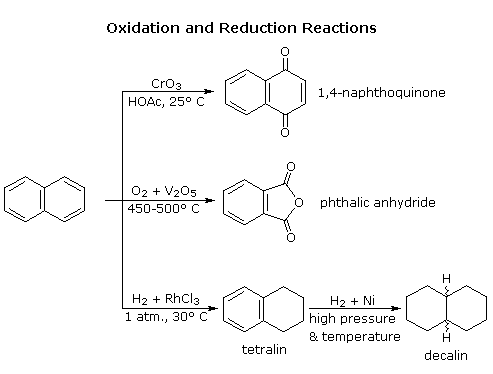
Naphthalene is more reactive than benzene, both in substitution and addition reactions, and these reactions tend to proceed in a manner that maintains one intact benzene ring. The following diagram shows three oxidation and reduction reactions that illustrate this feature. In the last example, catalytic hydrogenation of one ring takes place under milder conditions than those required for complete saturation (the decalin product exists as cis/trans isomers). Electrophilic substitution reactions take place more rapidly at C1, although the C2 product is more stable and predominates at equilibrium. Examples of these reactions will be displayed by clicking on the diagram. The kinetically favored C1 orientation reflects a preference for generating a cationic intermediate that maintains one intact benzene ring. By clicking on the diagram a second time, the two naphthenonium intermediates created by attack at C1 and C2 will be displayed.
The structure and chemistry of more highly fused benzene ring compounds, such as anthracene and phenanthrene show many of the same characteristics described above.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


