Aromatic Ions and Antiaromaticity
- Page ID
- 1197
Aromatic Ions
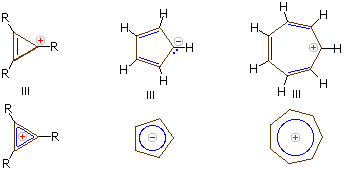
Carbanions and carbocations may also show aromatic stabilization. Some examples are:
The three-membered ring cation has 2 π-electrons and is surprisingly stable, considering its ring strain. Cyclopentadiene is as acidic as ethanol, reflecting the stability of its 6 π-electron conjugate base. Salts of cycloheptatrienyl cation (tropylium ion) are stable in water solution, again reflecting the stability of this 6 π-electron cation.
Antiaromaticity
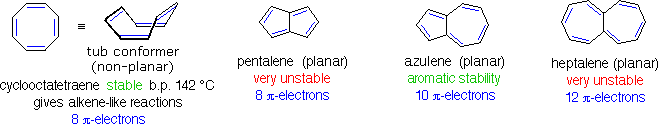
Conjugated ring systems having 4n π-electrons (e.g. 4, 8, 12 etc. electrons) not only fail to show any aromatic properties, but appear to be less stable and more reactive than expected. As noted above, 1,3,5,7-cyclooctatetraene is non-planar and adopts a tub-shaped conformation. The compound is readily prepared, and undergoes addition reactions typical of alkenes. Catalytic hydrogenation of this tetraene produces cyclooctane. Planar bridged annulenes having 4n π-electrons have proven to be relatively unstable. Examples of 8 and 12-π-electron systems are shown below, together with a similar 10 π-electron aromatic compound.
The simple C8H6 hydrocarbon pentalene does not exist as a stable compound, and its hexaphenyl derivative is air sensitive. The 12-π-electron analog heptalene has been prepared, but is also extremely reactive (more so than cyclooctatetraene). On the other hand, azulene is a stable 10-π-electron hydrocarbon that incorporates structural features of both pentalene and heptalene. Azulene is a stable blue crystalline solid that undergoes a number of typical aromatic substitution reactions. The unexpected instability of 4n π-electron annulenes has been termed "antiaromaticity". Other examples may be cited. Thus, all attempts to isolate 1,3-cyclobutadiene have yielded its dimer, or products from reactions with other compounds introduced into the reaction system. Similarly, cyclopentadienyl cation (4 π-electrons) and cycloheptatrienyl anion (8 π-electrons) show very high reactivity when forced to form.
Cyclooctatetraene is a fascinating compound. To see more of its chemistry Click Here.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry