Reducing Alkynes-The Reactivity of the Two π Bonds
- Page ID
- 920
Reactions between alkynes and catalysts are a common source of alkene formation. Because alkynes differ from alkenes on account of their two procurable π bonds, alkynes are more susceptible to additions. Aside from turning them into alkenes, these catalysts affect the arrangement of substituents on the newly formed alkene molecule. Depending on which catalyst is used, the catalysts cause anti- or syn-addition of hydrogens. Alkynes can readily undergo additions because of their availability of two π bonds.
Hydrogenation of an Alkyne
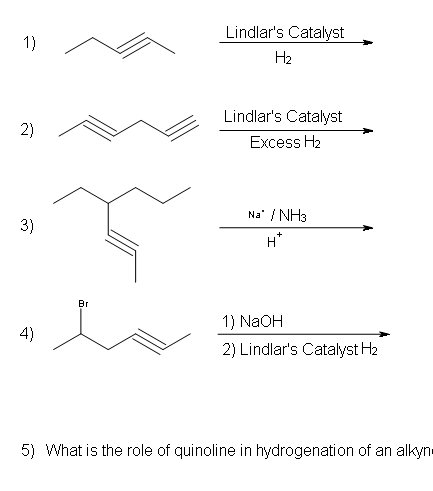
Alkynes can be fully hydrogenated into alkanes with the help of a platinum catalyst. However, the use of two other catalysts can be used to hydrogenate alkynes to alkanes. These catalysts are: Palladium dispersed on carbon (Pd/C) and finely dispersed nickel (Raney-Ni).
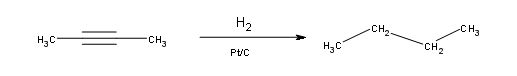
Hydrogenation of an Alkyne to a Cis-Alkene
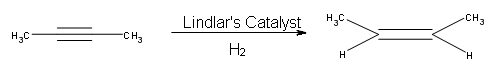
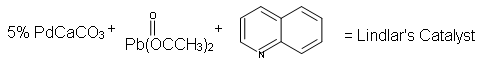
Because hydrogenation is an interruptible process involving a series of steps, hydrogenation can be stopped, using modified catalysts (e.g., Lindlar’s Catalyst) at the transitional alkene stage. Lindar’s catalyst has three components: Palladium-Calcium Carbonate, lead acetate and quinoline. The quinoline serves to prevent complete hydrogenation of the alkyne to an alkane. Lindlar’s Catalyst transforms an alkyne to a cis-alkene.
Lindlar's Catalyst:
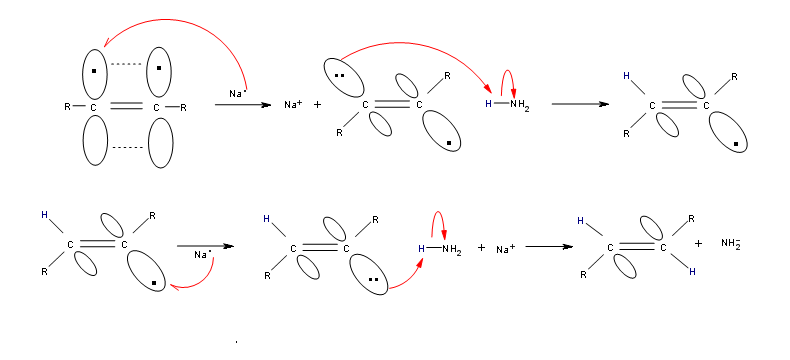
Hydrogenation of an Alkyne to a Trans-Alkene
Alkynes can be reduced to trans-alkenes with the use of sodium dissolved in an ammonia solvent. An Na radical donates an electron to one of the P bonds in a carbon-carbon triple bond. This forms an anion, which can be protonated by a hydrogen in an ammonia solvent. This prompts another Na radical to donate an electron to the second P orbital. Soon after this anion is also protonated by a hydrogen from the ammonia solvent, resulting in a trans-alkene.
References
- Ternay, Andrew L. Contemporary Organic Chemistry. 2nd ed. Saunders, 1979
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. New York: W.H. Freeman and Company, 2007.
- Williams, Jonathon M.J. Preparation of Alkenes: A Practical Approach. Illustrated ed. Oxford UP, 1996.
Problems
Contributors
- Ravjot Takhar (UCD)