Anti-Markovnikov Additions to Triple Bonds
- Page ID
- 914
Most Hydrogen halide reactions with alkynes occur in a Markovnikov-manner in which the halide attaches to the most substituted carbon since it is the most positively polarized. A more substituted carbon has more bonds attached to 1) carbons or 2) electron-donating groups such as Fluorine and other halides. However, there are two specific reactions among alkynes where anti-Markovnikov reactions take place: the radical addition of HBr and Hydroboration Oxidation reactions. For alkynes, an anti-Markovnikov addition takes place on a terminal alkyne, an alkyne on the end of a chain.
HBr Addition With Radical Yields 1-bromoalkene
The Br of the Hydrogen Bromide (H-Br) attaches to the less substituted 1-carbon of the terminal alkyne shown below in an anti-Markovnikov manner while the Hydrogen proton attaches to the second carbon. As mentioned above, the first carbon is the less substituted carbon since it has fewer bonds attached to carbons and other substituents. The H-Br reagent must also be reacted with heat or some other radicial initiator such as a peroxide in order for this reaction to proceed in this manner. This presence of the radical or heat leads to the anti-Markovnikov addition since it produces the most stable reaction. For more on Anti-Markovnikov additions:Radical Additions--Anti-Markovnikov Product Formation
The product of a terminal alkyne that is reacted with a peroxide (or light) and H-Br is a 1-bromoalkene.
Regioselectivity: The Bromine can attach in a syn or anti manner which means the resulting alkene can be both cis and trans. Syn addition is when both Hydrogens attach to the same face or side of the double bond (i.e. cis) while the anti addition is when they attach on opposite sides of the bond (trans).
.jpg?revision=1&size=bestfit&width=504&height=271)
Stereospecific Hydroboration Oxidation of Alkynes
Step 1: Hydroboration of terminal alkynes reacts in an anti-Markovnikov fashion in which the Boron attacks the less substituted carbon which is the least hindered. It is a stereospecific reaction where syn addition is observed as the hydroboration occurs on the same side of the alkyne and results in cis stereochemistry. However, a bulky borane reagent needs to be used to stop at the alkenyl-borane stage. Otherwise, a second hydroboration will occur. In this example, diisoamyl borane or HBsia2, a fairly large and sterically-hindered borane reagent, is used. Both of the alkyne's pi bonds will undergo hydroboration if BH3 (borane) is used by itself.
Step 2: Oxidation is the next step that occurs. The resulting alkylborane is oxidized to a vinyl alcohol due to the reaction with a hydroxide in a basic solution such as aqueous sodium hydroxide. A vinyl alcohol is an alcohol that has both an alkene and an -OH group. After the vinyl alcohol is formed, tautomerization takes place. Tautomerism is the interconversion of isomeric compounds due to the migration of a proton. The alcohol, which in this case is a terminal enol, spontaneously and rapidly rearranges due to tautomerism to become an aldehyde since the aldehyde form is much more stable than the enol.
For additional information on hydroboration oxidation: Hydroboration Oxidation
References
- Brown, Herbert C. Hydroboration With Supplement. New York: Addison-Wesley Pub Co, 1980.
- Dhillon, Ranjit S. Hydroboration and Organic Synthesis 9-Borabicyclo [3.3.1] nonane (9-BBN). New York: Springer, 2007.
- Minkin, V. I. Molecular design of tautomeric compounds. Dordrecht: D. Reidel Pub. Co., U.S.A. and Canada, 1988.
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry Structure and Function. New York: W. H. Freeman, 2005.
Outside Links
- http://en.Wikipedia.org/wiki/Hydroboration-oxidation
- Markovnikov's Rule: http://en.Wikipedia.org/wiki/Markovnikov%27s_rule
- For more on alkynes: www.ulm.edu/~junk/examkeys/pp230_7_ch8.ppt
Problems
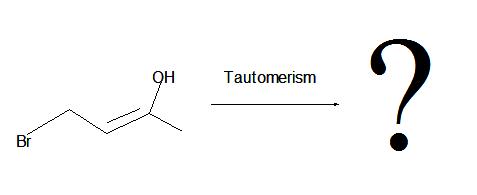
1. What is the product of this reaction?
.jpg?revision=1&size=bestfit&width=591&height=243)
2. What process causes the conversion of a vinyl alcohol to an aldeyde and what are some of its distinct features?
3. True or False: Only cis products are observed in radical H-Br additions to terminal alkynes.
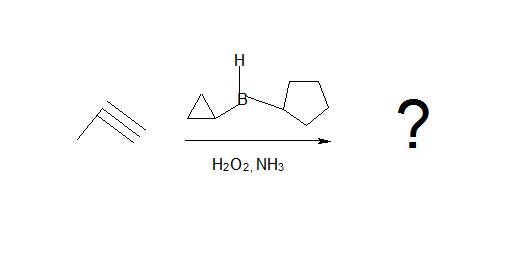
4. Explain why a bulky borane reagent is necessary for hydroboration oxidation reactions.
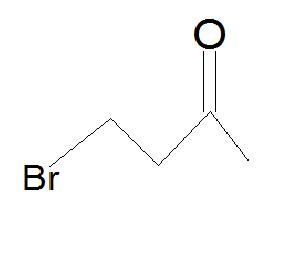
5. Draw the product.
Answers
1. Don't be confused by the borane reagent! Just remember, anytime there is a bulky borane reagent reacting with a terminal alkyne, the hydroboration oxidation reaction will occur and be proceeded by tautomerism which will produce an aldeyde as shown below.
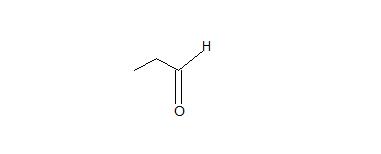
2. The interconversion of an enol or vinyl alcohol to an aldehyde is called tautomerism and it is very distinct since it is a spontaneous reaction that proceeds very quickly.
3. False. Both cis and trans products are produced as both syn and anti additions are observed.
4. If a small borane reagent is utilized, both pi bonds will be used and a second hydroboration will occur. This will break the double bond of the alkene and the aldehyde product will not be formed.
5.
Contributors
- Ali Alvandi