Alkenes by Dehydration of Alcohols
- Page ID
- 32755
Alkenes are usually prepared from either alcohols or haloalkanes (alkyl halides).
Dehydration of Alcohols
Alkenes are obtained by the dehydration of alcohols. The dehydration of alcohols can be affected by two common methods.
- By passing the vapors of an alcohol over heated alumina.
- By heating an alcohol with concentrated mineral acid, such as concentrated \(H_2SO_4\) or concentrated \(H_3PO_4\). Anhydrous zinc chloride can also be used as a dehydrating agent.
By passing the vapors of an alcohol over alumina (\(Al_2O_3\)) at 623 K (350°C).
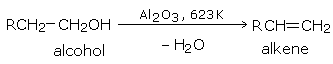
The order of the ease of dehydration of alcohols is: tertiary > secondary > primary. Secondary and tertiary alcohols are best dehydrated by dilute sulfuric acid.
By heating an alcohol with concentrated sulfuric acid at 453 K (180°C).
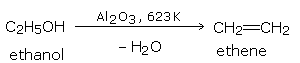
Other dehydrating agents like phosphoric acid and anhydrous zinc chloride may also be used.
Cyclohexanol on dehydration gives cyclohexene.
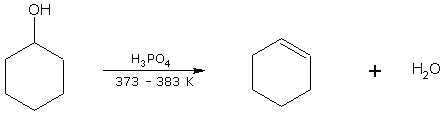
cyclohexanol cyclohexene
The loss of water from an alcohol to give an alkene does not occur in just one step; a series of steps are involved in the mechanism of dehydration of alcohols. In the dehydration reaction given above, the following steps are involved.
- First, the acid protonates (adding a proton or H+) the alcohol on the most electronegative atom, namely oxygen. This process is usually reversible.
- In the second step, the protonated alcohol loses water to give a positively charged species known as a carbonium ion or carbocation.
- Finally the carbonium ion loses a proton to give alkene.
The mechanism of dehydration of ethyl alcohol is described below.
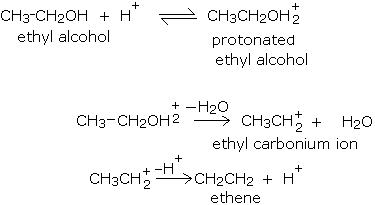
Contributors
Binod Shrestha (University of Lorraine)

