Hydroxylation
- Page ID
- 901
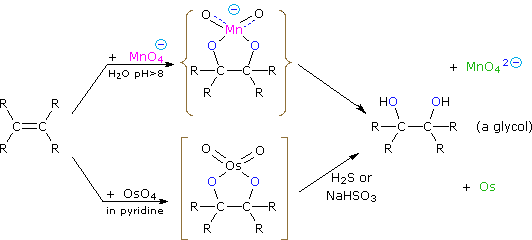
Dihydroxylated products (glycols) are obtained by reaction with aqueous potassium permanganate (pH > 8) or osmium tetroxide in pyridine solution. Both reactions appear to proceed by the same mechanism (shown below); the metallocyclic intermediate may be isolated in the osmium reaction. In basic solution the purple permanganate anion is reduced to the green manganate ion, providing a nice color test for the double bond functional group. From the mechanism shown here we would expect syn-stereoselectivity in the bonding to oxygen, and regioselectivity is not an issue.
When viewed in context with the previously discussed addition reactions, the hydroxylation reaction might seem implausible. Permanganate and osmium tetroxide have similar configurations, in which the metal atom occupies the center of a tetrahedral grouping of negatively charged oxygen atoms. How, then, would such a species interact with the nucleophilic pi-electrons of a double bond? A possible explanation is that an empty d-orbital of the electrophilic metal atom extends well beyond the surrounding oxygen atoms and initiates electron transfer from the double bond to the metal, in much the same fashion noted above for platinum. Back-bonding of the nucleophilic oxygens to the antibonding π*-orbital completes this interaction. The result is formation of a metallocyclic intermediate, as shown below.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry